
OUR NEW PROFILE IS: (NASDAQ: GNPX)
________________________
GNPX HAS CLOSED GREEN 9 OUT OF THE LAST, GAINING HUGE MOMENTUM
CHECK OUT THE INVESTOR PRESENTATION HERE
GNPX HAS ALREADY RECEIVED FAST TRACK DESIGNATION FOR ACCLAIM 2
BREAKING NEWS IN PLAY TODAY:
Genprex Announces U.S. Patent for REQORSA™ Immunogene Therapy in Combination with Immune Checkpoint Inhibitors to Treat Cancers
_________________________
Hello Everyone,
![Rodney Varner, CEO of Austin-based biotech company Genprex, stands at the podium during the closing bell ceremony for the Nasdaq exchange in 2018 following the company's initial public offering of stock. [Photo courtesy Genprex]](https://www.gannett-cdn.com/authoring/2020/02/24/NAAS/ghows-TX-ade062b1-031d-455e-8c47-8ec9f0909c2f-453ac4bb.jpeg?width=660&height=441&fit=crop&format=pjpg&auto=webp)
We have another explosive Nasdaq profile on the docket today. Our last one ran 11% from the open in just a couple of sessions.
This next one has a FREIGHT TRAIN of momentum behind it right now.
Pull up GNPX right away and get it on your screen.
It is already seeing some action premarket after they just dropped a strong press release this morning.
Genprex, Inc. is a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes. Genprex’s technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options. Genprex works with world-class institutions and collaborators to develop drug candidates to further its pipeline of gene therapies in order to provide novel treatment approaches. Genprex’s oncology program utilizes its unique, proprietary, non-viral ONCOPREX® Nanoparticle Delivery System, which the Company believes is the first systemic gene therapy delivery platform used for cancer in humans. ONCOPREX encapsulates the gene-expressing plasmids using lipid nanoparticles. The resultant product is administered intravenously, where it is then taken up by tumor cells that express proteins that are deficient. The Company’s lead product candidate, REQORSA™ (quaratusugene ozeplasmid), is being evaluated as a treatment for non-small cell lung cancer (NSCLC). REQORSA has a multimodal mechanism of action that has been shown to interrupt cell signaling pathways that cause replication and proliferation of cancer cells; re-establish pathways for apoptosis, or programmed cell death, in cancer cells; and modulate the immune response against cancer cells. REQORSA has also been shown to block mechanisms that create drug resistance. In 2020, the U.S. Food and Drug Administration (FDA) granted Fast Track Designation for REQORSA for NSCLC in combination therapy with AstraZeneca’s Tagrisso® (osimertinib) for patients with EFGR mutations whose tumors progressed after treatment with Tagrisso. In 2021, the FDA granted Fast Track Designation for REQORSA for NSCLC in combination therapy with Merck & Co’s Keytruda® (pembrolizumab) for patients whose disease progressed after treatment with Keytruda.
CATALYSTS
– Addressing unmet medical need in large markets through the buildout of a robust pipeline of new drug candidates and drug combinations.
– Leveraging a gene therapy platform; Genprex’s non-viral ONCOPREX® Nanoparticle Delivery System is designed to deliver a variety of therapeutic genes to fight multiple types of cancer.
– REQORSATM immunogene therapy, the first systemically delivered gene therapy used for cancer in humans, can be combined with top-selling cancer drugs and may improve their benefits.
– Genprex has demonstrated clinical achievement with REQORSA in two clinical trials, showing a favorable safety profile and evidence of efficacy in lung cancer.
– GPX-002, Genprex’s diabetes gene therapy, works to transform alpha cells in the pancreas into insulin producing beta-like cells. A Phase 1 clinical trial could be the first-ever gene therapy tested in humans for diabetes.
LOOK AT WHAT HAS BEEN GOING ON THE PAST FEW WEEKS WITH GNPX. IT BROKE OUT OF IT’S CHANNEL AND IS AGGRESSIVELY MOVING NORTH.
*****BREAKING NEWS THIS MORNING*****
Genprex Announces U.S. Patent for REQORSA™ Immunogene Therapy in Combination with Immune Checkpoint Inhibitors to Treat Cancers
August 16, 2022
Provides Protection for Therapeutic Combination in Acclaim-2 Phase 1/2 Clinical Trial
AUSTIN, Texas — (August 16, 2022) — Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that the United States Patent and Trademark Office (USPTO) has granted Genprex U.S. Patent No: 11,278,592 B2. The patent covers methods of using REQORSATM Immunogene Therapy in conjunction with immune checkpoint inhibitors, through 2038.
“This new patent provides critical protection for our REQORSA gene therapy in combination with a checkpoint inhibitor, for example an anti-PD1 antibody, to treat cancer. This is important as our ongoing Acclaim-2 Phase 1/2 clinical trial combines REQORSA™ with Keytruda®, an anti-PD1 antibody, to treat non-small cell lung cancer,” noted Thomas Gallagher, Esq., Senior Vice President of Intellectual Property and Licensing at Genprex. “We continue to strengthen our intellectual property portfolio and continue to build protection around our technology as it safeguards our gene therapy with target-specific combination therapy, is a deterrent to would-be competitors and creates value around our core competencies.”
Acclaim-2 is an open-label, multi-center Phase 1/2 clinical trial evaluating the Company’s lead drug candidate, REQORSA™ Immunogene Therapy, in combination with Keytruda® (pembrolizumab) in patients with late-stage non-small cell lung cancer (NSCLC) whose disease progressed after treatment with Keytruda. More information on Acclaim-2 can be found at www.clinicaltrials.gov Identifier NCT05062980.
In 2021, Genprex received U.S. Food and Drug Administration’s (FDA) Fast Track Designation for treatment of the Acclaim-2 patient population.
Keytruda® is a registered trademark of Merck & Co. and is its largest selling drug with 2021 sales of more than $17 billion.
The Acclaim-2 trial is a Phase 1/2 open-label, dose-escalation and clinical response study of REQORSA in combination with Keytruda in patients with advanced, metastatic non-small-cell lung cancer who have progressed after treatment with Keytruda. The Company anticipates enrolling patients at approximately 15 clinical sites and estimates that the Phase 1 portion of the Acclaim-2 trial will enroll up to 30 patients and the Phase 2 portion will enroll approximately 126 patients. Patients enrolled in the Phase 2 portion of the study will be randomized 2:1 to either REQORSA and Keytruda combination therapy or to chemotherapy (docetaxel with or without ramucirumab). Patients will be treated until disease progression or unacceptable toxicity is experienced. Patients must have histologically confirmed unresectable stage III or IV NSCLC (any histology) with radiological progression on Keytruda and an ECOG performance status of 0 to 1. Genprex expects to complete the Phase 1 portion of Acclaim-2 by mid-2023.
About REQORSA
REQORSA™ Immunogene Therapy (quaratusugene ozeplasmid) for non-small cell lung cancer (NSCLC) uses Genprex’s unique, proprietary ONCOPREX® Nanoparticle Delivery System, which is the first systemic gene therapy delivery platform used for cancer in human clinical trials. The active ingredient in REQORSA is the TUSC2 gene, a tumor suppressor gene. REQORSA consists of the TUSC2 gene encapsulated in a nanoparticle made from lipid molecules with a net positive electrical charge. REQORSA is injected intravenously and can specifically target cancer cells, which generally have a negative electrical charge. Once REQORSA is taken up into a cancer cell, the TUSC2 gene is expressed, and the TUSC2 protein is capable of restoring certain defective functions arising in the cancer cell. REQORSA has a multimodal mechanism of action whereby it interrupts cell signaling pathways that cause replication and proliferation of cancer cells, re-establishes pathways for programmed cell death, or apoptosis, in cancer cells, and modulates the immune response against cancer cells. REQORSA has also been shown to block mechanisms that create drug resistance.
Tagrisso® is a registered trademark of AstraZeneca plc and its largest selling drug with 2021 sales of over $5 billion.
_____________
2022 Genprex Shareholder Letter
May 5, 2022
To my fellow shareholders:
GENPREX CORPORATE UPDATE
Genprex has been off to a strong start for 2022. I am enthusiastic about our future as we continue to advance our leading edge gene therapy programs with the goal of extending hope and life to patients with serious disease and unmet need.
Large Markets and Unmet Need
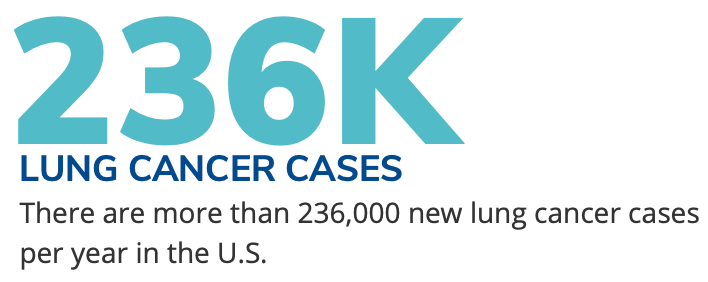
I believe that Genprex is attracting attention for the significantly large markets and unmet need that we target. Most gene therapy companies are focused on rare diseases, characterized by serious illness but small numbers of patients. In contrast, Genprex is developing gene therapies for diseases affecting very large patient populations with correspondingly large markets. Our initial targeted diseases are lung cancer and diabetes. Lung cancer continues to be the world’s leading cause of cancer death. Each year, lung cancer strikes about 236,000 people in the United States and 2 million people worldwide. The market for lung cancer therapeutics is estimated to exceed $48 billion by 2026. Type 1 and Type 2 diabetes together affect 37 million people in the United States, or 11% of the population.
We believe that the large patient populations and market sizes we are pursuing will become more widely understood and appreciated as we move forward, driving our growth and future success.
Second FDA Fast Track Designation.
2022 began with the announcement of our second Food and Drug Administration (FDA) Fast Track Designation (FTD). This second FTD is for use of our most advanced drug candidate, REQORSATM Immunogene Therapy, in combination with Merck and Co’s Keytruda® (pembrolizumab) in non-small cell lung cancer (NSCLC) patients whose disease progressed on Keytruda. Merck’s gross revenue from Keytruda exceeded $17 billion in 2021. This drug combination and patient population are the subject of our Acclaim-2 clinical trial. We opened this trial for enrollment in March 2022 and treated our first patient in April 2022.
Our first FDA Fast Track Designation was received in 2020 for use of REQORSA in combination with AstraZeneca’s Tagrisso® (osimertinib) in NSCLC patients whose disease progressed on Tagrisso. AstraZeneca’s gross revenues from Tagrisso exceeded $5 billion in 2021. This drug combination and patient population are the subject of our Acclaim-1 clinical trial. Patients are being enrolled and treated in the Acclaim-1 trial. Our first patient was treated in February 2022.
REQORSA consists of the TUSC2 gene inserted into a DNA plasmid and encapsulated in a nonviral delivery system made from lipid nanoparticles. REQORSA is injected intravenously where it specifically targets cancer cells. Once REQORSA is taken up into the cancer cell, the TUSC2 gene expresses the TUSC2 protein which has been shown to be capable of restoring certain functions that are often defective in the cancer cell. REQORSA has a multimodal mechanism of action whereby it:
- interrupts cell signaling pathways that cause replication and proliferation of cancer cells,
- re-establishes pathways for programmed cell death in cancer cells,
- modulates the immune response against cancer cells,
- blocks mechanisms that create drug resistance.




It should be noted that we do not seek to take market share from the companies whose drugs are combined with ours. Instead, success of REQORSA in combination with these drugs would expand the markets for these drugs. REQORSA in combination with Tagrisso or Keytruda could enable more patients to benefit from those drugs, potentially for longer periods of time, and may provide additional therapeutic benefits.
Expanding Pipeline
In early 2022, we added small cell lung cancer (SCLC) to our pipeline. SCLC represents about 13% of lung cancers. NSCLC, which is targeted in our Acclaim clinical trials, represents about 84% of lung cancers. Thus, with the addition of SCLC to our pipeline, we now target nearly 100% of the lung cancer market. We believe that this distinguishes REQORSA from almost all other lung cancer drugs in development because they only target subsets of lung cancer. By combining REQORSA with both Tagrisso and Keytruda, and including SCLC as well as NSCLC, our present program does target nearly all patients with lung cancer.
Expanding our pipeline continues to be a strategic priority. Because of this, we are continuing to sponsor research with the same major cancer research center that developed our REQORSA immunogene therapy. This includes new preclinical research exploring REQORSA’s potential to treat other cancer indications, and the use of REQORSA in combination with other cancer drugs.
Strengthened Leadership
We started 2022 stronger with the addition of two seasoned, innovative drug development executives to lead our clinical and manufacturing functions. In September 2021, Mark S. Berger, M.D. joined Genprex as Chief Medical Officer, and Hemant Kumar, Ph.D., CPM, EMBA joined Genprex as Chief Manufacturing and Technology Officer.
Dr. Berger is an oncologist and senior executive with 25 y ears of biotech and pharmaceutical company experience in the development of oncology therapeutics. He has successfully brought two cancer drugs through the regulatory process to approval and excels in strategic development, team management and collaborative leadership.
Dr. Kumar is a recognized global expert in Chemistry, Manufacture and Controls (CMC) Technical Development and GMP manufacturing. He has more than 25 years of experience leading global CMC and regulatory approval strategy for the accelerated development of innovative vaccines, biologics, advanced cell and gene therapy drug processes and product development under current GMP and licensing processes.
These two new executives are making major contributions to the advancement of our drug candidates.
Clinical Advisory Board
In 2021, we also formed a new Clinical Advisory Board, which is comprised of world renowned medical and clinical experts, to guide our clinical development programs in cancer and diabetes. The Clinical Advisory Board’s vast experience in clinical trials of cancer and diabetes therapies, combined with their expertise in consulting with small and large biotechnology companies, is invaluable as our drug candidates progress into and through the clinic.
The members of Genprex’s Clinical Advisory Board include:
- Michael Morse, MD, MHS, FACP – Dr. Morse is a Professor of Medicine in the Division of Medical Oncology and Professor in the Department of Surgery at Duke University Medical Center, Durham, NC. His clinical expertise includes management of gastrointestinal malignancies including colon, hepatobiliary, gastroesophageal and pancreatic cancer. His research expertise includes the development of targeted therapies including immunotherapies for cancer.
- Col. George E. Peoples, MD, FACS – Dr. Peoples served 30 years of active duty as a surgeon and research scientist in the military. He is the Founder and Director of the Cancer Vaccine Development Program (CVDP), which has been focused on the discovery, development, and clinical testing of cancer vaccines for more than 20 years. Four of the program’s cancer vaccines have been licensed for commercial development. Dr. Peoples currently serves as the CEO of Cancer Insight, LLC, CVDP’s commercial counterpart, which is a boutique cancer immunotherapy Contract Research Organization conducting multiple Phase 1 and 2 clinical trials. He also serves as Professor of Surgery at the Uniformed Services University of the Health Sciences and Professor (adjunct) of Surgical Oncology at MD Anderson Cancer Center (MDACC).
• Andrew B. Becker, MD, PhD – Dr. Becker is President and Founder of Becker Pharmaceutical Consulting, a market research, competitive intelligence and strategic planning consulting firm that provides analytic and strategy services to companies ranging from small biotechnology and medical device companies up to large multinational pharmaceutical companies on a global basis. Dr. Becker received both his medical degree and PhD in Molecular Pharmacology from Stanford University where his research focused on signaling pathways for the insulin and IGF– 1 receptors, deciphering the structure and function of the insulin degrading enzyme and its role in insulin processing.
REQORSA, Our Lead Gene Therapy Candidate in Oncology
Our lead therapeutic candidate is REQORSA Immunogene Therapy, which utilizes our unique, proprietary, non-viral ONCOPREX® Nanoparticle Delivery System, in combination with approved targeted therapies and immunotherapies, such as Tagrisso (osimertinib) and Keytruda (pembrolizumab) for treatment of lung cancer. We are conducting two clinical trials of REQORSA in lung cancer, which we have branded as Acclaim-1 and Acclaim-2.
Acclaim-1
In 2021, we initiated the Acclaim-1 clinical trial and in 2022 we dosed our first patient. Acclaim-1 is a Phase 1/2, open-label, dose escalation and clinical response study of the Company’s lead drug candidate, REQORSATM Immunogene Therapy, in combination with Tagrisso in patients with advanced, EGFR-mutant metastatic NSCLC whose disease progressed after treatment with Tagrisso. As previously mentioned, in 2020, we received U.S. Food and Drug Administration (FDA) Fast Track Designation for the treatment of the Acclaim-1 patient population.
We anticipate enrolling patients at approximately 15 clinical sites. We expect the Phase 1 portion of Acclaim-1 to enroll up to 18 patients in a dose escalation study to determine the maximum tolerated dose of the combination. The Phase 2 portion of the study is expected to enroll approximately 74 patients to be randomized 1:1 to receive either REQORSA and Tagrisso combination therapy or Tagrisso monotherapy. The primary endpoint of the Phase 2 portion of the trial is progression-free survival, which is defined as time from randomization to progression or death. An interim analysis will be performed at 25 events.
We are in the process of engaging and adding premier clinical trial sites to participate in our Acclaim-1 study. We expect the Phase 1 portion of Acclaim-1 to be completed by year end 2022.
Acclaim-2
At the end of 2021, we received FDA Fast Track Designation for the treatment of the Acclaim-2 patient population. We believe this second Fast Track Designation is an important step in accelerating the clinical development of REQORSA, and it is another indication of REQORSA’s potential to treat the unmet medical need of patients with late-stage NSCLC.
In March 2022, we opened Acclaim-2 for enrollment. I am pleased to announce that we dosed our first patient in Acclaim-2 in April 2022.
Acclaim-2 is an open-label, dose escalation and clinical response study of REQORSA in combination with Keytruda in patients with advanced, metastatic NSCLC whose disease progressed after treatment with Keytruda.
We anticipate enrolling patients at approximately 15 clinical sites. We expect the Phase 1 portion of Acclaim-2 to enroll up to 30 patients to determine the maximum tolerated dose of the combination of REQORSA and Keytruda. The Phase 2 portion of the study is expected to enroll approximately 126 patients to be randomized 2:1 to receive either REQORSA and Keytruda combination therapy or docetaxel and/or ramucirumab. The primary endpoint of the Phase 2 portion of the trial is progression-free survival, which is defined as time from randomization to progression or death. An interim analysis will be performed after 50 events.
We are in the contracting process with a number of additional prestigious cancer centers across the United States to participate in the Acclaim-2 study. We expect to complete the Phase 1 portion of Acclaim-2 by the end of the first quarter of 2023.
We look forward to generating data from these two clinical trials, and we believe that positive results in either or both of them would be a major step forward for lung cancer patients and create significant value for the Company.
___
2021 American Association of Cancer Research (AACR) Preclinical Data
At the 2021 AACR annual meeting, our collaborators presented positive preclinical data for the combination of REQORSA in combination with chemotherapy and immunotherapies, and in combination with targeted therapies, for the treatment of NSCLC at the 2021 American Association of Cancer Research (AACR) annual meeting.
These positive data provide further support for the therapeutic potential of REQORSA in NSCLC and for the study designs of Acclaim-1 and Acclaim-2.
The first presentation at AACR, entitled “TUSC2 immunogene therapy enhances efficacy of chemo-immune combination therapy and induces robust antitumor immunity in KRAS-LKB1 mutant NSCLC in humanized mice,” showed that the combination of immunotherapy (immune checkpoint blockade) and REQORSA demonstrated strong antitumor efficacy and induced robust antitumor immunity in KRAS-LKB1 (KL)-mutant NSCLC in clinically relevant humanized mouse models. It was also reported that REQORSA and immunotherapy and chemotherapy combinations were associated with strong efficacy in xenografts in humanized mice.
This poster and its data are relevant to our Acclaim-2 clinical trial which is combining REQORSA and Keytruda, an immunotherapy in advanced NSCLC patients whose disease progressed after treatment with Keytruda. The Acclaim-2 trial is investigating whether the synergy seen with REQORSA and immunotherapy in humanized mice will translate into clinical benefits in patients.
The second poster presentation at AACR, entitled “Overcoming resistance to Osimertinib [Tagrisso] by TUSC2 gene therapy in EGFR-mutant NSCLC,” showed synergistic antitumor efficacy of REQORSA combined with Tagrisso in EGFR mutant Tagrisso resistant NSCLC tumor xenografts. The TUSC2 gene is the active ingredient in REQORSA.
The data from the second poster is particularly important as it relates to our Acclaim-1 clinical trial combining REQORSA and Tagrisso. Approximately 17% of NSCLC patients of North American and European descent and approximately 30% to 50% of NSCLC patients of Asian descent have activating EGFR mutations. Identification of these patients is clinically important, as the optimal initial therapy for them is an EGFR tyrosine kinase inhibitor (TKI), such as Tagrisso. However, among those patients who are EGFR positive and benefit from EGFR TKI therapy, nearly all eventually become resistant to and ultimately no longer respond to EGFR TKI therapy, resulting in disease progression. For example, according to the FLAURA study, sponsored by AstraZeneca, the median time to tumor progression for lung cancer patients on Tagrisso is approximately 18 months.
This AACR poster data supports the study design for the Phase 2 portion of Acclaim-1 that will enroll patients whose disease progressed on Tagrisso. Those patients may receive local therapy for progressing lesions, and will then receive either continued Tagrisso or the combination of REQORSA and Tagrisso, which we believe may extend the benefit of EGFR TKI therapy for NSCLC patients. This has the potential to change the paradigm for treatment of NSCLC patients who are no longer benefitting from Tagrisso and could provide a new treatment for NSCLC patients in desperate need of new therapies.
Key Opinion Leader Event
REQORSA’s potential in lung cancer was featured in a 2021 Key Opinion Leader event sponsored by Alliance Global Partners. The event, titled, “Advances in Gene Therapy for Oncology: New Opportunities with REQORSATM (TUSC2),” was moderated by James Molloy, Managing Director, Equity Research at AGP and featured discussions with Andrew Becker, M.D., Ph.D. and Col. George Peoples, M.D., FACS, members of Genprex’s Clinical Advisory Board.
The presentation provided an overview of REQORSA and ONCOPREX and included discussions supporting the Company’s belief that REQORSA is the first systemic gene therapy used for cancer in human clinical trials. It also featured the large markets that REQORSA is addressing, as well as REQORSA’s potential to address a range of cancers. The discussion also reviewed highlights from Genprex’s two previous clinical trials, ONC-001, a REQORSA monotherapy clinical trial; and ONC-002, a combination of REQORSA and Roche’s Tarceva® in NSCLC. The KOLs also discussed the importance of the positive preclinical data presented at the 2021 AACR annual meeting that support the use of REQORSA in combination with immunotherapies and with targeted therapies for the treatment of NSCLC.
A replay of this discussion is available on our website. Expansion of Intellectual Property Portfolio
During 2021, we secured an amendment to the Patent and Technology License Agreement with a major cancer research center in Houston, Texas, that was originally executed in 2020. This amendment adds an additional portfolio of six patents and one patent application and related technologies to Genprex’s exclusive worldwide license. The newly licensed intellectual property includes methods for treating NSCLC by administration of REQORSA in conjunction with EGFR inhibitors or other anti-cancer therapies, in patients who are predicted to be responsive to REQORSA therapy.
We continue to expand our intellectual property protection for our arsenal of combination therapies for REQORSA as we develop a world-class intellectual property portfolio in the gene therapy space.
Gene Therapy for Diabetes
GPX-002 is a potentially curative diabetes gene therapy that was developed by lead researcher, Dr. George Gittes, of the University of Pittsburgh. In 2021, Genprex received the inaugural “License of the Year” award from the University of Pittsburgh Innovation Institute (UPII) in recognition of the advances made with our license from University of Pittsburgh toward progressing the development of GPX-002. We are honored to receive this recognition, considering the large number of cutting-edge technologies coming out of such a distinguished university and research center.
In addition, our collaborators at the University of Pittsburgh showcased our novel, preclinical diabetes gene therapy at the 2021 American Diabetes Association Scientific Sessions. In a video produced with the University of Pittsburgh Medical Center, sponsored by Genprex, attendees were able to learn more about this gene therapy approach for the treatment of both Type 1 and Type 2 diabetes.
Meanwhile, throughout the year Dr. Gittes and his team at the Rangos Research Center at UPMC Children’s Hospital of Pittsburgh continued preclinical studies of GPX-002 in nonhuman primates as they move toward first-in-human clinical trials.
We believe this diabetes gene therapy could hold the potential to provide long-term effectiveness for this debilitating disease and become a new treatment option for the millions of diabetes patients who now must rely on insulin replacement therapy.
Corporate Highlights
Enhancing REQORSA Manufacturing
To date, we have developed a robust manufacturing process for REQORSA through years of process development activities and the manufacturing technology has been successfully transferred from an academic setting to industry contract development and manufacturing organizations (CDMOs) using current Good Manufacturing Practices (cGMP) standards.
With the addition of Dr. Kumar to Genprex’s management, we are preparing for commercial readiness of REQORSA through the development of an integrated supply chain network of vendors. Under his leadership, we also are working to identify the most advanced manufacturing technologies in our industry so that we may leverage them and continue to be on the cutting-edge of gene therapy product development and manufacturing. These efforts will enable us to continue to support our clinical programs for REQORSA at multiple cancer centers across the United States and to prepare for future commercialization.
Strengthened Balance Sheet
In 2021, we strengthened our balance sheet by raising gross proceeds of $25 million in a registered direct offering priced at the market, with no warrants. As of December 31, 2021, we had $38.6 million in cash and no debt, which we believe, based on certain assumptions about our expenditures, would fund our operations into 2024.
A Strong Start to 2022
We have started 2022 strong by achieving many key milestones:
• Receipt of FDA Fast Track Designation for Acclaim-2
This Fast Track Designation is an important achievement in our efforts to accelerate clinical development of REQORSA and another indication of the potential of REQORSA to treat the unmet medical need of patients with late-stage NSCLC.

• Dosing the first patient in the Acclaim-1 clinical trial
This is an exciting milestone for Genprex which we believe brings us one step closer to achieving our goal to validate the potential benefits of REQORSA in NSCLC patients who have life-limiting disease and few treatment options.
- Opening enrollment and dosing the first patient in the Acclaim-2 clinical trial
We recently opened enrollment and dosed the first patient in Acclaim-2, our clinical trial of REQORSA incombination with Keytruda in late-stage NSCLC patients whose disease progressed on Keytruda. - Expansion of our clinical development pipeline to include small cell lung cancer
REQORSA may have the potential to treat small cell lung cancer, in addition to non-small cell lung cancer, thusmaking REQORSA a possible drug candidate for nearly the entire lung cancer market. - New preclinical data show the potential of our ONCOPREX® Nanoparticle Delivery System in treating colon cancerWe recently announced positive preclinical data showing that the ONCOPREX Nanoparticle Delivery System may be used to deliver tumor suppressor genes other than TUSC2, which is the active ingredient in REQORSA, to address multiple types of cancer. With this compelling data, Genprex is positioned to continue expanding our oncology programs and further explore use of our delivery system for other therapeutic genes, alone or in combination with other approved cancer therapies.As we continue to advance our clinical development strategies, I believe we are set to advance our gene therapies toward commercialization and to distinguish ourselves from other gene therapy companies. I am confident in Genprex’s technologies. REQORSA, ONCOPREX and GPX-002 have the potential to provide more effective therapies for very large patient populations with cancer and diabetes who are in desperate need for new treatment options.With numerous important milestones ahead of us, I am excited to see Genprex emerge as a leader in gene therapy. I believe in our potential to extend hope and life to patients and increase value for our shareholders.We thank you for your continued support of our Company.Sincerely,Rodney Varner
Chairman, President and Chief Executive Officer, Genprex
NEWS
Genprex Announces U.S. Patent for REQORSA™ Immunogene Therapy in Combination with Immune Checkpoint Inhibitors to Treat Cancers
August 16, 2022
Intellectual Property Protection for Therapeutic Combination in Acclaim-2 Phase 1/2 Clinical Trial
Genprex Announces Safety Review Committee Approves Dose Escalation in Acclaim-1 Phase 1/2 Trial of REQORSA™ in Combination with Tagrisso® in Non-Small Cell Lung Cancer
August 15, 2022
Recommends Advancing Acclaim-1 to Increased Dose in Second Cohort of Phase 1 Portion of Trial
Genprex to Participate in Next Generation Lipid-Based Nanoparticles Delivery Summit
July 13, 2022
Company Manufacturing Leads Selected as Thought Leaders to Discuss Advances in Lipid Nanoparticle Delivery Systems
Genprex to Present at Upcoming June Investor Conferences
June 2, 2022
Corporate Presentation to Highlight Company’s Gene Therapies for Cancer and Diabetes
Genprex to Present at Upcoming Investor Conference in May 2022
May 19, 2022
Corporate Presentation to Highlight Company’s Gene Therapies for Cancer and Diabetes
Genprex’s Chief Medical Officer to Be Featured as an Expert Panelist at the 33rd Annual Cancer Progress Conference
May 9, 2022
Live panel discussion will explore considerations with respect to positioning of cell-based and other emerging immunotherapy platforms in solid tumors
Genprex Issues Shareholder Letter and Provides 2022 Corporate Update
May 5, 2022
Company achieves major milestones in clinical development programs in 2022
Patient treatment in Acclaim-2 clinical trial commences
Genprex to Participate in Upcoming Investor and Industry Conferences in April 2022
April 18, 2022
Presentations to highlight the Company’s gene therapies for cancer and diabetes
Genprex Announces the Opening for Enrollment of its Phase 1/2 Acclaim-2 Clinical Trial of REQORSA™ Immunogene Therapy in Combination with Keytruda® to Treat Non-Small Cell Lung Cancer
March 31, 2022
Company Has FDA Fast Track Designation for Combination of REQORSA and Keytruda
Genprex to Participate in Upcoming Investor Conference in March
March 23, 2022
Investor presentation to highlight the Company’s gene therapies for cancer and diabetes
MANAGEMEnT
Check out the management team here: https://www.genprex.com/about/company-management/
Sincerely,
The Viral Stocks Team





