OUR NEW PROFILE IS: (NASDAQ: NMTC)
NeuroOne® Announces $3.5 Million Accelerated Milestone Payment from Zimmer Biomet for Evo® sEEG Electrode
Neurone® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
CHECK OUT THE INVESTOR PRESENTATION HERE
______________________________________
Hello Everyone,
Our last profile opened at $7.60 and ran to $8.80 pretty quick and was sitting above 8 again the next day before treading water at the profile price.
Some of these lower priced Nasdaq profiles have seen some serious volatility since the end of the Summer.
With that being said we have another exciting Nasdaq profile sitting under a buck with a chart full of wild swings.
Pull up NMTC Immediately.
The 52 week low is right around .50 and NMTC is sitting well above that but you will notice on the chart that this was a support level for a few months over the Summer so it will be interesting to see where it goes from it’s current levels.
NeuroOne Medical Technologies Corporation is a developmental stage company committed to providing minimally invasive and hi-definition solutions for EEG recording, brain stimulation and ablation solutions for patients suffering from epilepsy, Parkinson’s disease, dystonia, essential tremors, chronic pain due to failed back surgeries and other related neurological disorders that may improve patient outcomes and reduce procedural costs.
- Disruptive next-generation diagnostic electrodes based on game-changing technology developed in collaboration with Mayo Clinic, a shareholder of the Company.
- Strategic partnership with Zimmer Biomet (NYSE:ZBH, ~$24B mkt cap) to exclusively commercialize and distribute EVO® diagnostic electrodes; accelerated payment of $3.5 million received in August 2022.
- Potential to penetrate large addressable markets including epilepsy ($1+ billion) and Parkinson’s disease ($5+ billion), spinal cord stimulation ($10+ billion revenue), and other disorders such as motor function, hypertension, high blood pressure, depression, and pain.
- Multi-billion market opportunity for combination devices; potential for technology adaptation and entry into AI and machine learning markets.
- Platform technology with licensing potential for applications in urinary incontinence, pain management, hypertension, depression, and other related neurological disorders.
- Ample capital resources to support upcoming commercial and development catalysts including the launch of thesecond diagnostic electrode, the Evo® sEEG diagnostic line (if cleared by the FDA), and further development of a combination diagnostic and ablation electrode system.
- Leadership with deep expertise in medical device technology, marketing, and business development; world-class board of directors, esteemed scientific and physician advisory boards.
We are excited to announce:
Neurone® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day UseSee full press release here: https://t.co/Lbn4qjCfle#NMTC #N1MTC #NeuroOne pic.twitter.com/u4qa49YG0j
— NeuroOne Medical Technologies Corporation (@N1MTC) October 25, 2022
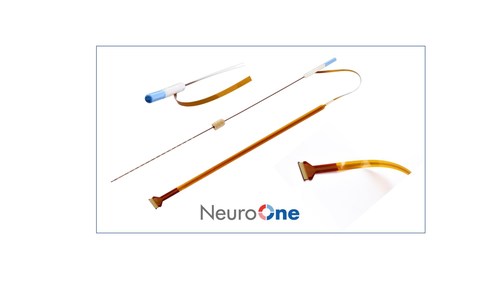
- Evo® Cortical ElectrodeNeuroOne offers a breakthrough thin-film electrode for the diagnosis of various neurological conditions. Evo’s high- definition, minimally invasive technology delivers major advantages over the limitations of legacy silicone-based electrodes, hampered by the lack of innovation and progress in electrode technology, that are still widely used today.o Over 7 times thinner and 8 times lighter than comparable silicone electrodes used in legacy products. o Flexibility, thinness, and reduced weight facilitates potential for minimally invasive placement.o Reduced inflammation as compared to silicon-based legacy electrodes based on published testing by Mayo.Evo cortical electrode technology has received 510(k) clearance from the FDA for recording, monitoring, and stimulating brain tissue for up to 30 days.
|
Evo® sEEG Electrode The Evo sEEG electrode technology offers stereoelectrocencephalography recording, spinal cord stimulation, brain stimulation and ablation solutions. The first clinical case using the Evo sEEG electrode was recently performed by Dr. Robert Gross at Emory University for intraoperative brain mapping at the subsurface level of the brain. The sEEG electrode has received FDA 510(k) clearance from the FDA for use of less than 24 hours. A 510(k) for Evo sEEG electrode technology for up to 30 day use has been submitted to the FDA. |
|
|
Brain Monitoring – Understanding What Allows Us To Sense, Move, Think And Feel
Neurons
86 BILLION NEURONS
Neurons communicate through electrical and chemical signals. The specific properties of the neurons composing a brain area and their connections with neurons in other brain areas, define the function of a brain area (e.g. language, vision, movement).
Monitoring Neural Activity
THEY CAN RECORD ELECTRICAL SIGNALS
There are different ways to record and monitor neural activity. Their solution is to provide electrodes that are placed on top of or within the brain and capture the activity of a group of neurons located underneath/around each contact. The signals recorded are called intracranial electroencephalogram (iEEG) or electrocorticography (ECoG) signals. The shape, amplitude and frequency of these electrical signals provide a readout of the activity of that brain area.
Monitoring Neurological Disorders
Neurological diseases may be associated with disturbances in neural activity and connectivity between brain areas. The changes in the shape, amplitude and frequency of recorded electrical signals may be used to identify areas with altered activity.
Epilepsy
Epilepsy is the disease associated with spontaneously recurring seizures. A seizure is a sudden, uncontrolled electrical disturbance in the brain. It can cause changes in behavior, movements or feelings, and in levels of consciousness. There are different types of seizures and their characterization and classification helps guide the treatment.
There are more than twenty seven FDA-approved drugs for the treatment of seizures. However, in ~1/3 of the patient’s medication fails to control their seizures. These patients are candidates for surgical options.
Evo® Cortical Electrode
Cortical or subdural electrodes are used in electrocorticography (ECoG) or intracranial electroencephalography (iEEG) surgeries to monitor, record and stimulate the surface of the brain for up to 30 days. The company’s Evo cortical electrode portfolio consists of various contact configurations of strip and grid electrodes. (Rx only)
The Evo Advantage
Decreased Immunological Response – The Evo Electrode is over 7 times thinner than a silicone electrode. The flexibility and reduced volume should reduce pain and edema.
The Evo’s polyimide substrate has properties like increased flexibility, reduced volume, and decreased immunological response, which should reduce signal artifacts.
Single Tail Design – The single thin tail design allows the implanted electrode tail to be tunneled through one incision which should reduce infection risk and procedure time.
Reduced Cable Management – A disposable cable assembly is sent with each Evo Electrode as an electrode kit, removing the need to source the correct cables for each electrode being used in surgery. Also, hospital resources are freed from the management of sterilizing and storing individual electrode cable assemblies.
How NeuroOne’s Thin Film Electrode Technology is Different
In comparison to currently available technologies, our electrodes are manufactured with polyimide thin film. Our electrodes are designed to reduce trauma to the brain by allowing a less invasive implant due to the flexibility, thinness and reduced weight of the electrode.
Furthermore, the potential to significantly increase the resolution of brain recordings may enable the usage of powerful computing techniques, such as machine learning and artificial intelligence.
Caution: US Federal law restricts this device to sell by or on the order of a physician
Two FDA-Cleared Devices and Counting
Are you beginning to understand that NeuroOne is not just “another” MedTech company? In fact, their Evo Cortical and sEEG Electrodes are both FDA approved!
What they are doing right now has incredible implications for the future of the medical industry.
So much so that the technologies below could be commonplace and in wide use across the globe.
NeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
Company focused on manufacturing ramp for commercialization in partnership with Zimmer Biomet
EDEN PRAIRIE, Minn., Oct. 25, 2022 /PRNewswire/ — NeuroOne Medical Technologies Corporation (Nasdaq: NMTC) (NeuroOne or the Company), a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market its Evo sEEG Electrode technology for temporary (less than 30 days) use with recording, monitoring, and stimulation equipment for the recording, monitoring, and stimulation of electrical signals at the subsurface level of the brain.
The Company’s Evo Cortical and sEEG Electrodes are a portfolio of hi-definition thin film electrodes. Potential advantages include increased signal clarity and reduced noise; better tactile feedback during insertion into brain tissue; and faster order fulfillment due to an automated manufacturing process.
As previously reported, NeuroOne is also advancing a pipeline of therapeutic electrode technologies for brain tissue ablation and chronic stimulation use for DBS (deep brain stimulation) and spinal cord stimulation for chronic back pain. These therapeutic electrode technologies represent addressable markets valued between $500 million and $6 billion.
NeuroOne® Reports Third Quarter Fiscal Year 2022 Financial Results and Provides Corporate Update
EDEN PRAIRIE, Minn., Aug. 11, 2022 /PRNewswire/ — NeuroOne Medical Technologies Corporation (NASDAQ: NMTC) (“NeuroOne” or the “Company”), a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, today announces its operating results for the third quarter of fiscal year 2022 ended June 30, 2022.
Third Quarter and Recent Business Updates
- Zimmer Biomet agreed to an accelerated $3.5M milestone payment for Evo® sEEG electrode; Zimmer received a warrant to purchase 350,000 shares of NeuroOne’s common stock at $3.00 per share.
- Re-submitted special 510(k) to FDA for <30 day use for the Evo sEEG electrode.
- Completed ablation system prototypes, including RF generator from RBC Medical and NeuroOne’s electrodes and accessories, ahead of schedule and successfully tested the first fully integrated sEEG/RF ablation system.
- Achieved major milestone with first sEEG electrode case for intraoperative brain mapping at the subsurface level of the brain performed at Emory University.
- Qualified second source for manufacturing for both the Evo cortical and sEEG electrodes.
- Completed validation and placed orders for a new cable assembly which will provide significant cost reduction.
- Established advisory board of leading anesthesiologists and neurosurgeons for spinal cord stimulation program.
- Completed design verification to extend shelf life for Evo cortical electrodes to three years.
- Presented at the HC Wainwright Global Investment Conference and the NobleCon 18 Investor Conference.
- Exhibited the Evo Cortical electrode family in partnership with Zimmer Biomet at the American Association of Neurological Surgeons Annual Scientific Meeting and the American Society for Stereotactic and Functional Neurosurgery Biennial Meeting.
- The first peered-reviewed paper presenting the biocompatibility results for NeuroOne subdural thin film electrodes was published in the Frontiers of Neuroscience journal on April 29, 2022.
- The Company’s Evo sEEG implantation accuracy study was accepted as a podium presentation at the Biennial Meeting of the World Society for Stereotactic & Functional Neurosurgery, to be held in South KoreaSeptember 4-7 2022.
Dave Rosa, CEO of NeuroOne commented, “The Company made great strides in the third fiscal quarter of 2022 and subsequent six weeks. Significant progress was made in testing our Evo sEEG electrode and on August 9th we re-submitted to the FDA a special 510(k) seeking clearance for <30 day use. In addition, we were excited to have the first Evo sEEG case performed by Dr. Robert Gross of Emory University for less than 24 hour use. We were also successful in reducing costs by completing validation of a new cable assembly and extending shelf life of the Evo Cortical electrode product lines. A backup supplier for our electrodes was also qualified providing additional insurance for our supply chain. Regarding our ablation system which we have named OneRF™ we successfully completed bench top testing for the entire system.
Finally, we are excited that Zimmer Biomet agreed to an accelerated $3.5Mmilestone payment for the Evo® sEEG electrode.” Rosa continued, “This amendment to our agreement provides NeuroOne with near term capital without the need for a highly dilutive financing and reinforces our ongoing partnership with Zimmer Biomet.”
Upcoming Targeted Milestones
- Evo sEEG Depth Diagnostic Electrode
- OneRF(RF ablation system)
- Partner with a research organization to develop electrode for new clinical indication.
- Chronic Use Electrode
Third Quarter of Fiscal 2022 Financial Results
Product revenue was $32,000 in the third quarter of fiscal 2022, compared to product revenue of $40,000 in the third quarter of fiscal 2021. Collaboration revenue was delayed in the third quarter of fiscal 2022 due to the FDA decision, compared to collaboration revenue of $17,000 in the third quarter of fiscal 2021. Collaboration revenue is derived from the Zimmer Development Agreement and represents the portion of the upfront initial development fee payment eligible for revenue recognition as of June 30, 2022.
Total operating expenses in the third quarter of fiscal 2022 were $2.8 million, compared with $3.0 million in the prior year third quarter. R&D expense in the third quarter of fiscal 2022 was $1.2 million, compared with $0.9 million in the same period of fiscal 2021. SG&A expense in the third quarter of fiscal 2022 was $1.5 million, compared with $2.1 million in the prior year third quarter.
Net loss for the third quarter of fiscal 2022 was $2.8 million, compared to a net loss of $3.0 million in the third quarter of fiscal 2021.
As of June 30, 2022, the Company had cash of approximately $10.2 million, compared to $6.9 million as of September 30, 2021, the end of the Company’s most recent fiscal year. The Company had no debt outstanding as of June 30, 2022.
NeuroOne® Announces $3.5 Million Accelerated Milestone Payment from Zimmer Biomet for Evo® sEEG Electrode
Amendment provides Zimmer Biomet with 350,000 warrants with exercise price of $3.00 per share
EDEN PRAIRIE, Minn., Aug. 3, 2022 /PRNewswire/ — NeuroOne Medical Technologies Corporation (NASDAQ: NMTC) (“NeuroOne” or the “Company”), a medical technology company focused on improving surgical care options and outcomes for patients suffering from neurological disorders, today announced that the Company entered into an amendment to its Exclusive Development and Distribution Agreement with Zimmer Biomet, Inc. (“Zimmer”) that will provide the Company with a $3.5 million accelerated payment within 10 business days which relates to certain milestone payments. In addition, Zimmer Biomet will receive a Warrant to purchase 350,000 shares of the Company’s common stock, with an exercise price of $3.00 per share.
Dave Rosa, Chief Executive Officer of NeuroOne, states, “I want to thank Zimmer for all their support to date and their confidence in our business, technology and future endeavors. This agreement accomplishes multiple objectives for NeuroOne, most importantly by providing additional capital to our balance sheet in the short-term without the need for a highly dilutive financing, and further reinforcing our ongoing partnership with Zimmer.”
Brian Hatcher, President of the Trauma, CMFT, Foot and Ankle Division of Zimmer said, “We look forward to continuing the relationship with NeuroOne as we advance our mission to alleviate pain and improve the quality of life for people around the world.” Under the Exclusive Development and Distribution Agreement signed by both parties in July 2020, Zimmer Biomet has exclusive global distribution rights to distribute the Company’s Cortical and sEEG diagnostic electrode technology.
NEWS
Oct 25, 2022 09:00am
NeuroOne® Receives FDA 510(k) Clearance to Market its Evo® sEEG System for Less than 30 Day Use
Sep 22, 2022 09:00am
NeuroOne Reports Inducement Grants Under Nasdaq Listing Rule 5635(c)(4)
Aug 11, 2022 04:00pm
NeuroOne® Reports Third Quarter Fiscal Year 2022 Financial Results and Provides Corporate Update
Aug 09, 2022 09:00am
NeuroOne® Submits Special 510(k) to FDA for Evo® sEEG Electrode
Aug 04, 2022 09:00am
NeuroOne® to Report Third Quarter Fiscal Year 2022 Financial Results on August 11
Aug 03, 2022 09:00am
NeuroOne® Announces $3.5 Million Accelerated Milestone Payment from Zimmer Biomet for Evo® sEEG Electrode
Jul 12, 2022 09:00am
NeuroOne® Announces First Clinical Case Using Evo® sEEG Electrode
Jun 02, 2022 08:47am
NeuroOne® Issues Letter to Stockholders in Connection With 2022 Annual Meeting of Stockholders
May 19, 2022 09:30am
NeuroOne® to Present Its Breakthrough Technology for Monitoring Neural Activity at the H.C. Wainwright Global Investment Conference on May 25
May 16, 2022 04:30pm
NeuroOne Provides Evo® sEEG Update
May 12, 2022 04:05pm
NeuroOne Reports Second Quarter Fiscal Year 2022 Financial Results and Provides Corporate Update
May 03, 2022 09:00am
NeuroOne® to Report Second Quarter Fiscal Year 2022 Financial Results on May 12
Apr 18, 2022 10:00am
NeuroOne® to Present its Disruptive Next-Generation Diagnostic Electrode Technology at the NobleCon18 Investor Conference on April 20, 2022
Apr 05, 2022 04:00pm
NeuroOne Reports Inducement Grant Under Nasdaq Listing Rule 5635(c)(4)
MANAGMENT






Sincerely,
The Viral Stocks Team



 On
On 




