 OUR NEW PROFILE IS: (NASDAQ: SPRC)
OUR NEW PROFILE IS: (NASDAQ: SPRC)
CHECK OUT THE INVESTOR PRESENTATION HERE
SCISPARC LTD. ANNOUNCES INITIAL POSITIVE RESULTS FOR THE COCAINE ADDICTION TREATMENT OF CLEARMIND MEDICINE INC
__________________________
Hello Everyone,
I hope that you were able to watch out last Nasdaq profile. It exploded out of the gate and ran to highs of 19% from the opening trade.
We have another exciting Nasdaq company that we want you to pay attention to for today’s session.
Pull up SPRC immediately.
SciSparc’s Board of Directors Provides Strategic Decision
TEL AVIV, Israel, June 09, 2022 (GLOBE NEWSWIRE) — SciSparc Ltd. (Nasdaq: SPRC), a specialty clinical-stage pharmaceutical company focusing on the development of therapies to treat disorders of the central nervous system (the “Company” or “SciSparc”), today announced that the Company’s board of directors resolved to initiate a process to review potential strategic transactions with the goal of maximizing shareholder value.
Potential strategic transactions to be explored and evaluated during the review process may include additional investments in innovative companies, acquisitions, a strategic partnership with one or more parties, or the licensing of one or more of the Company’s proprietary technologies or indications. If any such transaction results in equity consideration, the Company may consider making dividend in kind distributions, subject to applicable law and regulations, to its shareholders.
Pending any decision to undertake any such strategic transaction, the Company will continue its development activities in accordance with its existing business strategy.
“Our cash position provides us the opportunity to carefully consider a range of potential strategic alternatives designed to maximize shareholder value,” said Oz Adler, SciSparc’s Chief Executive Officer. “As we assess potential external strategic alternatives, we continue to seek to create value through the development of unique clinical-stage treatments for the central nervous system, including Alzheimer’s disease, Tourette syndrome and more. We plan to continue our trials according to the FDA guidelines and explore our new path with psychedelic pharmaceutical treatments.”
ABOUT SCISPARC
SciSparc’s proprietary compounds capitalize on the biochemistry of receptors that specialize in modulating the central nervous system to create therapeutics that mitigate the adverse symptoms associated with CNS disorders.
Our primary platforms focus on the endocannabinoid system. The SciSparc pipeline composes both FDA – approved, and New Chemical Entities (NCEs) which we believe have strong potential to be indicated for targeted conditions, with no existing or sufficiently effective therapies.
Enhancing existing compounds with our proprietary technologies, we have developed novel therapies that demonstrate in clinical trials heightened bioavailability, significantly improved efficacy, lower dosage requirements, better safety profiles and a reduction in side effects.
CATALYSTS
- Proprietary Technology with cutting-edge drug combinations addressing large global unmet medical needs
- Two Phase 2 Assets addressing multi-billion markets and a Pending Phase 3* Clinical Trial
- Attractive valuation & well capitalized to execute 2022 objectives
- Key strategic partners, including leading universities, medical centers and KOLs
- Preparing to file Investigational New Drug (IND) application in pain
- Top-line pre-clinical SE study results in Status Epilepticus expected in the second half of 2022

2021- 2022 PRE-CLINICAL & CLINICAL ACHIEVEMENTS
- SCI–110 for Alzheimer’s disease and Agitation – Recruited first patient for the Company’s Phase IIa
- Entered an agreement with two clinical sites: Hannover Medical School in Hannover, Germany, and Tel- Aviv Sourasky Medical Center, in Tel-Aviv, Israel, to further the Company’s Phase IIb clinical study for SCI-110 for patients suffering from Tourette Syndrome
- Entered into agreement with The Sheba Fund for Health Services and Research, to perform a pre- clinicalstudyforthe evaluationoftheCompany’sSCI-210drugdevelopmentprogram,forthe treatment of Status Epilepticus (SE)
- Received positive top-line results for the Company’s proprietary compound, SCI-160, in a controlled pre-clinical trial on Neuropathic and Post-operative pain
ROBUST IP PORTFOLIO
SciSparc has built an intellectual property portfolio which currently comprises three granted U.S. patents and pending patent applications in six families, all focused on disorders of the central nervous system. Currently, our drug candidates are designed to treat Tourette Syndrome, Autism Spectrum Disorder, Epilepsy, Obstructive Sleep Apnea, Alzheimer’s Disease and Agitation, and Pain.
Our research and development efforts are focused on creating a proprietary and unique combination of products, some available already in the market (CannAmide™), products in the accelerated regulatory path of 505 (b)(2) application (SCI-110, SCI-210) and
products that constitute a new chemical entity (SCI-160) in order to bring different products to market at different timelines and potentially enable us to generate immediate revenues for the company. augmenting FDA-approved natural and synthetic cannabinoids in combination with our proprietary compounds and technologies to create alternate therapies that potentiate the effects of cannabinoids and target the receptors implicated in modulating the central nervous system.
SCI-110, our proprietary drug candidate, containing Dronabinol (FDA approved synthetic form of THC), with the endocannabinoid palmitoylethanolamide (PEA).
Designed to stimulate cannabinoid receptors across the Central Nervous System and inhibit the metabolic degradation of endocannabinoids in order to improve uptake of THC, the expected benefits of SCI-110 are an increase in efficiency of oral administration, and in turn a decrease in dosage requirements, side effects and adverse events.
This product is being developed under the accelerated regulatory path of 505 (b)(2) application focused on augmenting FDA-approved natural and synthetic cannabinoids to create alternate therapies that potentiate the effects of cannabinoids and target the receptors implicated in modulating the central nervous system.
This approach qualifies us for access to the FDA’s 505 (b)(2) regulatory strategy, created to facilitate the submission of novel drug candidates that meet specific criteria to the FDA for review. The 505 (b)(2) application provides us with several advantages as compared to a typical New Drug Application, including potential; lower risk and development costs, and a potentially expedited time to market.
Indications currently being investigated for treatment with SCI-110 include:
– Tourette Syndrome- (TS)
– Obstructive Sleep Apnea (OSA)
– Alzheimer’s Disease and Agitation
Our proprietary drug candidate containing cannabidiol (CBD), a non-psychoactive cannabinoid, and PEA.
This product is initially being developed under the regulation of the Israeli Medical Cannabis Agency (IMCA) – the agency that leads the regularization of the medical cannabis field in Israel and is the first of its kind in the world. It is a complex, unique, innovative and original process. Conducting clinical trials and development under the regulation of the HQR ostensibly enables rapid and specific registration processes in a track that is unique to Israel.
The company intends to further develop the product for markets outside Israel as well. Indications currently being investigated for treatment with SCI-210 include
– Autism Spectrum Disorder (in clinical trials)
– Status Epilepticus – a form of seizures that are severe and sometimes fatal. This indication is currently investigated in pre-clinical settings.
SCI-160 is an innovative, proprietary synthetic CB2 receptor agonist created, among others, for the treatment of pain and is currently in pre-clinical studies. The CB2 receptor agonist used in this formulation – HU-433 – was invented and synthesized by Professor Raphael Mechoulam, Ph.D., Chairman of the SciSparc Scientific Advisory Board, and is protected under a patent granted in the U.S. and Europe.
CannAmide™ is an immediate unique palmitoylethanolamide (PEA) oral formulation for the reduction of chronic pain and inflammation. PEA is a cannabinoid mimetic lipid molecule found throughout the body, including the central nervous system. Similar to cannabinoids, PEA has been shown to have neuroprotective, anti-inflammatory, analgesic and anti-convulsant properties.
CannAmide is currently available in tablet form, with each dose containing 400mg active pharmaceutical ingredient. It has been designated a product license issuance from the Natural and Non-prescription Health Products Directorate (NNHPD) from Health Canada, for sale as a supplement within the nutraceuticals market.
THE CENTRAL NERVOUS SYSTEM
:max_bytes(150000):strip_icc()/brain_spinal_cord-57fe96b15f9b5805c26d5072.jpg)
The central nervous system (CNS) is the complex collection of nerve tissues that controls the activities of the body, comprising the brain and spinal cord. Disorders of the CNS include a broad category of conditions in which the brain does not function as it should, limiting health and the ability to function. These disorders may be the result of damage from an infection, inherited metabolic disorders, a degenerative condition, stroke, brain tumor, or arise from multiple unknown factors.
Most CNS disorders cannot be cured, and many have associated comorbidities. Symptoms can put a severe strain on the quality of life, interfering with everyday activities and functioning; they can be embarrassing, and in more extreme cases, debilitating. The best option for most patients is to control or limit the impact of the disease through a range of therapies from medical to surgical treatment.
THE ENDOCANNABINOID SYSTEM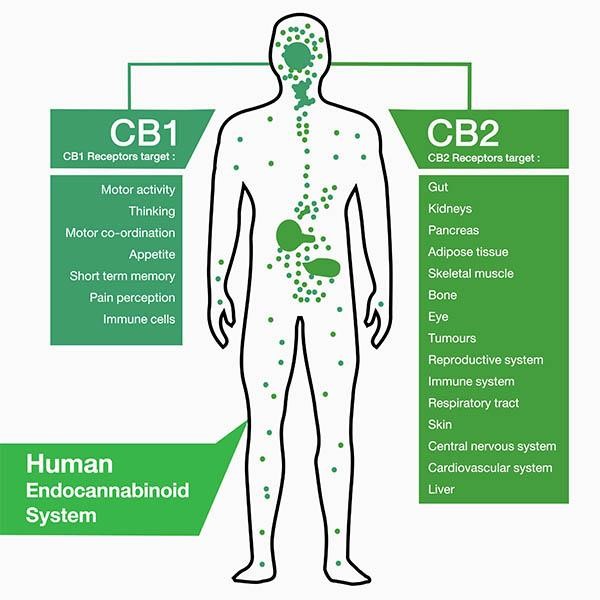
The endocannabinoid system (ECS) is a neurotransmitter network tasked with maintaining homeostasis throughout the body. Essentially a biochemical communication system, the ECS comprises endogenous cannabinoids and receptors. These endogenous cannabinoids, or endocannabinoids, are produced by multiple cell types within the CNS.
The main ECS receptors — CB1 and CB2 — are located throughout the body; CB1 primarily within brain cells and CB2 across the CNS. These receptors have been implicated in the modulation of neuroinflammatory, neurodegenerative and psychiatric conditions, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, amyotrophic lateral sclerosis, stroke, epilepsy, anxiety and depression.
Because the ECS acts with such precision, its interconnection with the CNS makes modulation of the ECS a therapeutic target for treating disorders of the CNS. And because cannabinoids, whether endogenous, plant based, or synthetic, impact the ECS equally, they are an obvious target for our continuing research and drug development efforts.
SCISPARC LTD. ANNOUNCES INITIAL POSITIVE RESULTS FOR THE COCAINE ADDICTION TREATMENT OF CLEARMIND MEDICINE INC.

SCISPARC AND CLEARMIND RECENTLY ANNOUNCED THE FILING OF A PATENT APPLICATION RELATED TO TREATING COCAINE ADDICTION
TEL AVIV, Israel, June 07, 2022 (GLOBE NEWSWIRE) — SciSparc Ltd. (NASDAQ: SPRC), a specialty clinical-stage pharmaceutical company focusing on the development of therapies to treat disorders of the central nervous system (the “Company” or “SciSparc”), today announced that initial positive pre-clinical results for treatment for cocaine addiction using MEAI, a novel psychedelic molecule of Clearmind Medicine Inc. (“Clearmind”) (CSE: CMND), (OTC: CMNDF), (FSE: CWY0).
Pursuant to SciSparc last announcement on June 2, 2022, whereby the Company announced the collaborated filing of a provisional patent application related to treating cocaine addiction with Clearmind based on SciSparc’s CannAmide™ compound and Clearmind’s MEAI, the pre-clinical trials show possible positive results which may positively affect our collaboration.
“We are excited about Clearmind’s results, in light of the potential synergistic effect between SciSparc’s CannAmide compound and Clearmind’s MEAI, as previously demonstrated in joint studies, which reflected the dose dependency effect. We plan to continue to investigate these findings, which for the first time point to a potential treatment for cocaine addiction. Since commencing the collaboration with Clearmind, we have been able to present successful results for our proprietary combination treatment” commented Oz Adler, SciSparc’s Chief Executive Officer. “It is our intention to further investigate the effect of this treatment on different addictions and other binge behaviors”.
The pre-clinical trial was led by Professor Gal Yadid and his team from the Gonda Multidisciplinary Brain Research Center located at Bar Ilan University (Ramat Gan, Israel) and was designed to evaluate the possible reward-like effects of MEAI. Four different doses of MEAI were compared to cocaine in the conditioned place preference (CPP) model in male Sprague-Dawley rats. Rats received either MEAI or cocaine. Based on statistically significant reductions in the reward effect as compared to cocaine, it was determined that MEAI is not rewarding. Additionally, some rats that received cocaine were treated with MEAI on the test day to determine if MEAI has a treatment-like effect. Rats were conditioned with cocaine and on the day of the test were treated with MEAI. The results obtained suggest MEAI’s ability to abolish cocaine induced conditioned place preference. The results of this ground-breaking research show potential for the first dedicated cocaine addiction treatment.
NEWS
TUE JUN 7TH, 2022
- GlobeNewswire8:30 AM
SCISPARC LTD. ANNOUNCES INITIAL POSITIVE RESULTS FOR THE COCAINE ADDICTION TREATMENT OF CLEARMIND MEDICINE INC.
THU JUN 2ND, 2022
- GlobeNewswire8:30 AM
SCISPARC LTD. AND CLEARMIND MEDICINE INC. COLLABORATION YIELDS ANOTHER PATENT APPLICATION FOR THEIR PROPRIETARY PSYCHEDELIC COMBINATION TO TREAT COCAINE ADDICTION
WED JUN 1ST, 2022
- GlobeNewswire4:07 PM
SCISPARC LTD. ANNOUNCES CLOSING OF $10 MILLION PRIVATE PLACEMENT PRICED AT-THE-MARKET
TUE MAY 31ST, 2022
- GlobeNewswire8:30 AM
SCISPARC TO COLLABORATE WITH POLYRIZON FOR BRAIN TARGETING USING INTRANASAL HYDROGEL SYSTEMS
FRI MAY 27TH, 2022
- GlobeNewswire9:15 AM
SCISPARC ANNOUNCES PRICING OF $10 MILLION PRIVATE PLACEMENT PRICED AT-THE-MARKET
THU MAY 26TH, 2022
- GlobeNewswire8:30 AM
SCISPARC ANNOUNCES ETHICS COMMITTEE APPROVAL TO CONDUCT A PHASE IIB TRIAL IN TOURETTE SYNDROME
TUE MAY 24TH, 2022
- GlobeNewswire8:30 AM
SCISPARC AND CLEARMIND MEDICINE INC. COLLABORATION YIELDS POSITIVE RESULTS FOR ITS PSYCHEDELIC COMBINATION TREATMENT
TUE MAY 10TH, 2022
- GlobeNewswire8:30 AM
CLEARMIND MEDICINE INC. AND SCISPARC LTD., COLLABORATION YIELDS NEW PROVISIONAL PATENT APPLICATION FOR PSYCHEDELIC COMBINATION TREATMENT FOR BINGE BEHAVIORS
- GlobeNewswire8:30 AM
SCISPARC LTD. AND CLEARMIND MEDICINE INC. COLLABORATION YIELDS NEW PATENT APPLICATION FOR PSYCHEDELIC COMBINATION TREATMENT FOR BINGE BEHAVIORS
MON MAY 2ND, 2022
- GlobeNewswire8:41 AM
SCISPARC FILES ANNUAL REPORT FOR YEAR ENDED DECEMBER 31, 2021 AND PROVIDES CORPORATE UPDATE
THU MAR 31ST, 2022
- GlobeNewswire9:30 AM
SCISPARC ANNOUNCES CLOSING OF NEW JV TARGETING DISCOVERY OF POTENTIAL DRUGS FOR CANCERS AND INFECTIOUS DISEASES
WED MAR 30TH, 2022
- GlobeNewswire8:30 AM
PROF. AVI SCHROEDER JOINS SCISPARC’S SCIENTIFIC ADVISORY BOARD TO SUPPORT THE COMPANY’S DEVELOPMENT OF SCI-160 FOR TREATING PAIN
THU MAR 17TH, 2022
- PR Newswire8:00 AM
SCISPARC ANNOUNCES POSITIVE RESULTS FROM PSYCHEDELIC-BASED PRE-CLINICAL TRIAL
THU MAR 10TH, 2022
- PR Newswire8:30 AM
SCISPARC LAUNCHES NEW JV TARGETING DISCOVERY OF POTENTIAL DRUGS FOR CANCERS AND INFECTIOUS DISEASES
TUE MAR 8TH, 2022
- PR Newswire8:30 AM
SCISPARC ENTERS COLLABORATION FOR THE DEVELOPMENT OF INNOVATIVE PSYCHEDELIC-BASED PHARMACEUTICALS
TUE FEB 22ND, 2022
- PR Newswire9:00 AM
SCISPARC TO PRESENT AT AEGIS VIRTUAL CONFERENCE
THU JAN 27TH, 2022
- PR Newswire8:30 AM
SCISPARC PRESENTS NEW LEADERSHIP LINEUP
TUE JAN 18TH, 2022
- PR Newswire8:02 AM
SCISPARC ADVANCES ITS PHASE IIB CLINICAL TRIAL IN PATIENTS WITH TOURETTE SYNDROME WITH ITS PROPRIETARY DRUG CANDIDATE SCI-110
TUE JAN 11TH, 2022
- PR Newswire8:30 AM
SCISPARC EXPLORING PSYCHEDELICS AS POSSIBLE AVENUE TO EXPAND ITS IP PORTFOLIO
THU JAN 6TH, 2022
- PR Newswire9:00 AM
SCISPARC ANNOUNCES RECRUITMENT OF FIRST PATIENT FOR ITS PHASE IIA CLINICAL TRIAL IN ALZHEIMER’S DISEASE
TUE JAN 4TH, 2022
- PR Newswire8:44 AM
SCISPARC ISSUED U.S. PATENT FOR ITS CORE TECHNOLOGY THAT TREATS CENTRAL NERVOUS SYSTEMS DISORDERS
___________________________
TOP MANAGEMENT
AMITAY WEISS
Chairman
Mr. Weiss has served as our Chief Executive Officer between August 2020 and January 2021 and on our Board of Directors since August 2020. He is founder and Chief Executive Officer of Amitay Weiss Management Ltd. Prior to forming his company, he held several positions at Bank Poalei Agudat Israel Ltd., most recently as Vice President of Business Marketing & Development. He currently chairs and serves as director on the boards of several public companies. Mr. Weiss earned his B.A. in Economics from New England College, and his M.B.A. and LL.B from Ono Academic College in Israel – a branch of the University of Manchester.
OZ ADLER, CPA
Chief Executive Officer and Chief Financial Officer
Mr. Adler joined the Company in September 2017, and since April 2018 serves as the Company Chief Financial Officer .Mr. Adler has a wide variety of managerial, financial, tax and accounting experience. Prior to joining the Company, Mr. Adler was employed as a CPA at Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global. He currently chairs and serves as director on the boards of several private and public companies. Mr. Adler holds a B.A. in Accounting and Business Management from The College of Management, Israel.
ADI ZULOFF-SHANI, PHD.
Chief Technologies Officer.
Dr. Zuloff-Shani joined the Company in February 2016, bringing more than 20 years of experience as a research and development executive in the bio-tech industry and launching start-ups in the healthcare industry. Dr. Zuloff-Shani brought 2 products from bench to market and is currently leading the development of several pharmaceutical products designated to the U.S., EU and Israeli markets. Prior to joining us, Dr. Zuloff-Shani served as Vice President Development at Macrocure Ltd. (Nasdaq: MCUR) where she led all research and development activities. Dr. Zuloff-Shani earned her Ph.D. in Human Biology and Immunology from Bar-Ilan University, Israel.
Sincerely,





