OUR NEW PROFILE IS: NASDAQ: CTXR
Strong balance sheet with $65.4 million in cash and cash equivalents as of December 31, 2021
Download the Investor presentation HERE
*****BREAKING NEWS THIS MORNING*****
Citius Pharmaceuticals to Accelerate Phase 3 Mino-Lok Trial by Expanding Trial Sites Internationally
_______________________________
Hello Everyone,
The market is about to open shortly and we have CTXR on our radar.
This is a company that you are going to want to take a look at.
Citius Pharmaceuticals, Inc. (Citius) is a late-stage biopharmaceutical company focused on the development and commercialization of first-in-class critical care products, with a diversified pipeline of anti-infectives in adjunct cancer care, stem cell therapy and unique prescription products. Three of its four pipeline candidates would be the first and only prescription treatments in their indications, if approved by the FDA. A Phase 3 pivotal superiority trial is currently underway for its lead product candidate, Mino-Lok®, an antibiotic lock solution to salvage infected central venous catheters (CVCs) of patients with catheter-related bloodstream infections (CRBSIs). Mino-Lok® was granted Fast Track designation by the FDA and would be the first and only FDA-approved treatment to salvage infected CVCs. Through its subsidiary, NoveCite, Inc., Citius is developing a novel proprietary mesenchymal stem cell (i-MSC) treatment derived from induced pluripotent stem cells (iPSCs) for acute respiratory conditions. Citius’s two additional product candidates are Halo-Lido, potentially the first and only FDA-approved prescription treatment for hemorrhoids, and Mino-Wrap, potentially the first and only to prevent infection in tissue expanders and breast implants post mastectomy.
*****BREAKING NEWS THIS MORNING*****
Citius Pharmaceuticals to Accelerate Phase 3 Mino-Lok Trial by Expanding Trial Sites Internationally
Additional sites to support trial completion by end of 2022
CRANFORD, N.J. , May 6, 2022 /PRNewswire/ — Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) (Nasdaq: CTXR), a late-stage biopharmaceutical company developing and commercializing first-in-class critical care products, today announced that it has selected Biorasi, LLC (“Biorasi”), a global clinical research organization (CRO), to help expand the Company’s Phase 3 Mino-Lok trial to additional sites outside the United States. If approved, Mino-Lok would be the first-and-only antibiotic lock solution FDA-approved to salvage infected central venous catheters (CVCs) causing catheter-related blood stream infections (CRBSIs).
“Citius is pleased to collaborate with Biorasi to expand the Mino-Lok trial to include international clinical sites, as originally planned. This complements efforts underway in the U.S. by our lead CRO, Medpace, to drive recruitment. We paused our ex-U.S. strategy as COVID-19 spread across the globe and hospitals halted non-COVID trials. With the COVID-19 pandemic receding, we believe there is now an opportunity to access additional sites and plan to leverage Biorasi’s track record of quickly ramping up sites around the world to recruit clinical trial subjects outside the U.S. CRBSIs remain a critical unmet need globally with millions of patients requiring sterile central venous catheters to receive life-saving therapies,” stated Leonard Mazur, Chairman and CEO of Citius.
“We are committed to continuing to recruit patients until we reach the minimum required trial events, as per FDA guidance and outlined in our trial protocol to achieve statistically significant results. This will enable us to optimize the potential of a successful New Drug Application (NDA) submission. We believe our efforts to establish trial sites outside the U.S., combined with our ongoing initiatives, which have driven a recent increase in study enrollment at our U.S. sites, will assist us in reaching the necessary events to complete the trial by the end of this year,” added Mazur.
About the Mino-Lok Phase 3 Trial
The Mino-Lok Phase 3 pivotal superiority trial (NCT02901717) is a multi-center, randomized, open-label, blinded study to determine the efficacy and safety of Mino-Lok (MLT), a novel antibiotic lock therapy that combines minocycline with edetate disodium. The primary endpoint for this study is the time (in days following randomization) to a catheter failure event between randomization and test of cure (TOC) (Week 6) in the Intent-to-Treat (ITT) Population. Additional secondary endpoints will be assessed including microbiological eradication and clinical cure.
Subjects in the Mino-Lok arm receive one MLT dose daily with a dwell time of two to four hours for a total of seven doses. For subjects in the Control arm, the investigator determines the antibiotic used in the lock, dose, dwell time, and number of days of administration based on institutional standards or Infectious Diseases Society of America (IDSA) guidelines.
About Biorasi, LLC
Biorasi is a customer-focused, full-service, contract research organization (CRO) that delivers fast and flexible solutions across global clinical trials to maximize speed-to-market for its sponsors. As the leader in neurology, nephrology, dermatology, and the rare and urgent disease market, Biorasi sets new benchmarks for speed, agility, and quality in patient enrollment, decentralized trials, and data transparency
Citius Pharmaceuticals, Inc. Provides Business Update, Highlights Upcoming Milestones
CRANFORD, N.J., May 3, 2022 /PRNewswire/ — Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) (Nasdaq: CTXR), a late-stage biopharmaceutical company developing and commercializing first-in-class critical care products, today provided a business update for the first quarter ended March 31, 2022 and reported on recent corporate developments and upcoming milestones.
Recent Highlights and Upcoming Milestones
- Citius anticipates filing a biologics license application (BLA) for I/ONTAK® with the U.S. Food and Drug Administration (FDA) in the second half of 2022;
- Topline data from the Phase 3 study of cancer immunotherapy I/ONTAK are consistent with the previously-approved formulation of denileukin diftitox (ONTAK), and there are no new safety signals;
- Halo-Lido Phase 2b trial initiated in April 2022 with last patient enrollment anticipated by the end of 2022;
- Phase 3 Mino-Lok® trial proceeding without modification as recommended by the independent data monitoring committee (DMC) following all three DMC reviews;
- Mino-Lok Phase 3 trial completion anticipated by end of 2022; and,
- Pre-clinical development ongoing for Citius’ Mino-Wrap and induced mesenchymal stem cell programs.
“2022 is a year of important catalysts for Citius as we continue to make progress on multiple fronts. We recently released topline results for I/ONTAK which were consistent with the prior FDA-approved and marketed formulation of denileukin diftitox (ONTAK). In preparation for a planned BLA submission in the second half of 2022, we are marshalling the necessary manufacturing and commercial resources to support the application, and ultimately a successful launch,” stated Leonard Mazur, Chairman and CEO of Citius.
“Our Mino-Lok program continues to advance in accordance with the recommendations of the independent data monitoring committee, which advised us to continue with the trial as planned, following each of its three data reviews. We remain encouraged by the positive signal conveyed by the DMC guidance to proceed. Coupled with the recent ramp up in patient recruitment following an easing of COVID-related hospital restrictions, we believe our efforts to increase engagement with existing trial sites and to onboard additional sites will continue to drive trial enrollment and enable us to achieve the necessary trial events to support statistically significant results,” added Mazur.
“In April, we initiated our Phase 2b Halo-Lido trial for the treatment of hemorrhoids. By the end of 2022, we expect to complete trial enrollment. A data readout will follow upon validation and analysis of the information provided in the electronic patient reported outcome tool (ePRO) designed with guidance from the FDA. The results will be used to design the Phase 3 trial. As this product would ultimately be marketed directly to consumers, rather than to targeted physician and hospital groups like our other pipeline candidates, we will evaluate alternatives to optimize the value of this asset as we advance the program. Citius considers all strategic alternatives to maximize the value of our portfolio, individually and collectively, on an ongoing basis. We believe we remain well capitalized to advance our programs through multiple catalysts this year, and we plan to continue building long-term value in the business by focusing on execution,” concluded Mr. Mazur.
2022 Achieved and Anticipated Catalysts
- Report Topline results of I/ONTAK Phase 3 trial (April 2022)
- Initiate Halo-Lido Phase 2b trial (April 2022)
- Submit I/ONTAK BLA application (2H 2022)
- Complete enrollment in Mino-Lok Phase 3 trial (end of 2022)
- Complete enrollment in Halo-Lido Phase 2b trial (end of 2022)
Citius Pharmaceuticals, Inc. Reports Fiscal First Quarter 2022 Financial Results and Provides Business Update
Topline results of Pivotal Phase 3 trial in cancer immunotherapy I/ONTAK for the treatment of cutaneous T-cell lymphoma expected 1H 2022; BLA submission planned for 2H 2022
Mino-Lok® Phase 3 trial progressed despite Covid-19-related recruitment challenges
CRANFORD, N.J., Feb. 10, 2022 /PRNewswire/ — Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) (Nasdaq: CTXR), a late-stage biopharmaceutical company dedicated to the development and commercialization of first-in-class critical care products with a focus on oncology, anti-infective products in adjunct cancer care, unique prescription products, and stem cell therapies, today reported business and financial results for the first fiscal quarter of 2022 ended December 31, 2021.
Fiscal Q1 2022 Business Highlights and Subsequent Developments
- Pivotal Phase 3 trial of I/ONTAK (E7777) completed in December 2021 with topline results anticipated in the first half of 2022 and a biologics license application (BLA) submission planned in the second half of 2022;
- Mino-Lok® Phase 3 trial completion anticipated in 2022; and,
- Regulatory, manufacturing, clinical and commercial capabilities expanded to support late-stage pipeline with the addition of seasoned executives with extensive pharmaceutical industry experience:
- Catherine Kessler MS – EVP, Regulatory Affairs
- Kelly Creighton, PhD – EVP, Chemistry, Manufacturing and Controls
- Kevin Carey – VP, Program Management
- Preeti Singh, MD – Medical Director
Financial Highlights
- Cash and cash equivalents of $65.4 million as of December 31, 2021;
- R&D expenses were $5.5 million for the first quarter ended December 31, 2021, compared to $6.2 million for the first quarter ended December 31, 2020;
- G&A expenses were $2.9 million for the first quarter ended December 31, 2021, compared to $1.7 million for the first quarter ended December 31, 2020;
- Stock-based compensation expense was $0.9 million for the first quarter ended December 31, 2021, compared to $0.3 million for the first quarter ended December 31, 2020; and,
- Net loss was $9.2 million, or ($0.06) per share for the first quarter ended December 31, 2021, compared to a net loss of $8.1 million, or ($0.15) per share for the first quarter ended December 31, 2020.
“We anticipate 2022 will be a year of important catalysts for Citius. The timeline for the I/ONTAK program remains on track, with topline results anticipated in the first half of 2022, followed by a planned BLA filing in the second half of the year. Moreover, the FDA confirmed that no pediatric study will be required for I/ONTAK, further de-risking this asset,” stated Myron Holubiak, President and Chief Executive Officer of Citius Pharmaceuticals.
“Covid-19 continues to pose a challenge to the Mino-Lok® Phase 3 trial. We remain committed to completing the trial this year and believe we are well-positioned to continue our efforts as Covid-19 infections and hospitalizations subside, restrictions are lifted and the overall environment for clinical trials improves. These efforts include active engagement with our existing sites, and evaluation of additional trial sites. We continue to believe that there is a significant unmet medical need to salvage catheters so that critically ill patients need not undergo the painful and costly removal and replacement of a central venous line. Our primary focus remains to execute a plan that ensures we have a robust dataset that maximizes the potential success of Mino-Lok®,” added Mr. Holubiak.
“To further support the launch of our two late-stage product candidates, I/ONTAK and Mino-Lok®, if approved, and to advance our other pipeline programs, we have added several key regulatory, clinical, commercial and manufacturing industry veterans to our team. Their expertise will help propel our activities to bring these important products to market, and our strong balance sheet continues to support these efforts. We look forward to sharing our value-creating milestones with our stakeholders in the coming months,” concluded Mr. Holubiak.
Key Recent Hires
Catherine Kessler, MS – EVP, Regulatory Affairs
Ms. Kessler is a well-respected biotech executive with more than 25 years of experience in the pharmaceutical industry, including 20 years of experience in regulatory affairs and 16 years of expertise in managing regulatory affairs and operations activities supporting early and late-stage product development in multiple therapeutic areas. She has prepared regulatory submissions for the US FDA, EMEA and other regulatory authorities for investigational drugs. Catherine’s deep expertise in developing regulatory paths to market for unique investigational products, engaging health authorities through complex stages of clinical development, tactical aspects of regulatory applications, and efficient resourcing of application-related activities will allow her to successfully chart the regulatory paths for each of the pipeline programs at Citius.
Kelly Creighton, PhD – EVP, Chemistry, Manufacturing and Controls
Mr. Creighton is a senior regulatory affairs and quality assurance expert with nearly two decades of experience in biopharmaceuticals, pharmaceuticals, advanced therapies, including gene and cellular therapies, and combination products. He joined Citius from Clinipace Worldwide, a leading global contract research organization. As head of global CMC regulatory activities for investigational products, he has led teams throughout North America, Europe and the Asia Pacific region overseeing submissions and negotiations with regulatory authorities, as well as biosafety and environmental agencies in each of these regions. Kelly has directed the implementation of multiple CMC development plans including: contract manufacturing organization selection, product manufacturing, analytical development, product characterization, specification establishment, container closure systems and stability requirements. Twenty products for which he prepared regulatory marketing applications (NDAs, ANDAs, and BLAs) were approved in the US and EU.
Kevin Carey – VP, Program Management
Mr. Carey is a seasoned pharmaceutical executive with more than 20 years of experience in complex global pharmaceutical project management, and more than 10 years of experience in combination drug/device development. Kevin has managed all phases of the pharmaceutical drug development lifecycle including discovery and development, preclinical research, clinical research, and FDA drug review and approval, including seven NDA submissions and approvals throughout his career. Mr. Carey joined Citius from Dr. Reddy’s Laboratories where he was a Senior Director and head of the Program and Alliance Management Office, and was integral to the I/ONTAK (E7777) program.
Preeti Singh, MD – Medical Director
Dr. Singh is an accomplished clinical strategy and development leader with more than a decade of experience in drug development from proof-of-concept studies to Phase 3 trials and life cycle management in the areas of oncology, dermatology, neurology, and pediatric and adult gastroenterology. She brings diverse and well-rounded experience in medical affairs, drug commercialization and strategy, with extensive knowledge of new drug approval and regulatory compliance, to the newly formed role at Citius. Dr. Singh joined Citius from Dr. Reddy’s Laboratories where she was the Subject Matter Expert on I/ONTAK (E7777).
FIRST QUARTER ENDED DECEMBER 31, 2021 Financial Results:
Liquidity
As of December 31, 2021, the Company had $65.4 million in cash and cash equivalents and no debt.
As of December 31, 2021, the Company had 146,012,169 common shares issued and outstanding.
The Company estimates that its available cash resources will be sufficient to fund its operations through March 2023.
Research and Development (R&D) Expenses
R&D expenses were $5.5 million for the fiscal quarter ended December 31, 2021, compared to $6.2 million for the fiscal quarter ended December 31, 2020. The decrease of $0.7 million is primarily due to a $4.8 million decrease in research and development expenses related to our proposed novel cellular therapy for ARDS offset by increases in R&D expenses related to I/ONTAK, Mino-Lok®, Halo-Lido and Mino-Wrap. During the three months ended December 31, 2020, we expensed a $5,000,000 license fee paid to Novellus.
We expect that research and development expenses will increase in fiscal 2022 as we continue to focus on our Phase 3 trials for Mino-Lok® and I/ONTAK, progress the Halo-Lido product candidate, and continue our research and development efforts related to ARDS and Mino-Wrap.
General and Administrative (G&A) Expenses
G&A expenses were $2.9 million for the fiscal quarter ended December 31, 2021, compared to $1.7 million for the fiscal quarter ended December 31, 2020. The increase of $1.2 million is primarily due to costs associated with additional compensation costs for new employees and performance bonuses. General and administrative expenses consist primarily of compensation costs, professional fees related to our capital raising activities, corporate development services, and investor relations.
Stock-based Compensation Expense
For the fiscal quarter ended December 31, 2021, stock-based compensation expense was $0.9 million as compared to $0.3 million for the prior year period. The increase primarily reflects expenses related to new grants made by Citius to employees, directors and consultants.
Net loss
Net loss was $9.2 million, or ($0.06) per share for the fiscal quarter ended December 31, 2021, compared to a net loss of $8.1 million, or ($0.15) per share for the fiscal quarter ended December 31, 2020. The increase in net loss is primarily due an increase in general and administrative expenses.
CTXR PIPELINE: FOUR ACTIVE PROGRAMS
MINO-LOK®
Mino-Lok® is an antibiotic lock solution used to treat patients with catheter-related bloodstream infections (CRBSIs) and central line 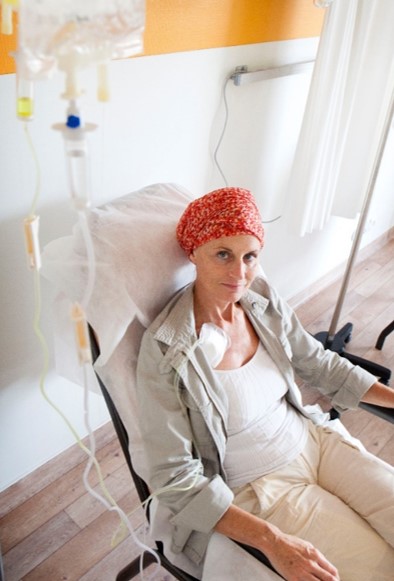 associated bloodstream infections (CLABSIs). CRBSIs/CLABSIs are life-threatening conditions, especially in cancer patients receiving therapy through central venous catheters (CVCs) and in hemodialysis patients where venous access presents a challenge.
associated bloodstream infections (CLABSIs). CRBSIs/CLABSIs are life-threatening conditions, especially in cancer patients receiving therapy through central venous catheters (CVCs) and in hemodialysis patients where venous access presents a challenge.
Mino-Lok® is intended to salvage the CVC, avoiding the need to remove and replace the infected catheter. Currently, there are few alternatives to removing and replacing a CVC once it becomes infected. Studies show that removal and reinsertion of CVCs have a 15% to 20% complication rate, including pneumothorax, misplacement, and arterial puncture. Mino-Lok® is being developed to address the complications, discomfort and cost of CVC removal and replacement. The are currently no FDA-approved products to salvage infected CVCs.
Program Highlights
- Mino-Lok® is the first & only therapy under investigation to salvage infected CVCs
- In a Phase 2b trial, Mino-Lok® demonstrated a 100% efficacy rate in salvaging colonized CVCs; Mino-Lok® had no significant adverse events compared to an 18% serious adverse event rate when infected CVCs were removed and replaced. Learn more…
- A multicenter Phase 3 pivotal superiority trial is currently underway. Learn more…
- Mino-Lok® was granted QIDP and Fast Track designation by the FDA and has patent protection through 2024 and formulation patent protection through 2036
- Citius licensed the worldwide rights to Mino-Lok® from The University of Texas MD Anderson Cancer Center.
How it works
Mino-Lok® contains a proprietary combination of minocycline, edetate (disodium EDTA), and ethyl alcohol, all of which act synergistically to break down bacterial biofilms, eradicate the bacteria, provide anti-clotting properties to maintain patency in CVCs, and salvage the indwelling catheter. Mino-Lok® is used in two-hour locking cycles, allowing the CVC to be used for its intended purposes for the remaining 22 hours each day.
HALO-LIDO
Prescription strength topical for symptomatic hemorrhoid treatment
Halo-Lido (CITI-002) is a proprietary topical formulation of halobetasol and lidocaine that is intended to provide anti-inflammatory and anesthetic relief to individuals suffering from hemorrhoids. In the United States, hemorrhoids affect nearly 5% of the population, with approximately 10 million patients annually reporting symptoms.
Program Highlights
- There are no FDA-approved prescription products on the market for hemorrhoids
- Citius’ halobetasol and lidocaine formulation could become the first FDA-approved prescription product to treat hemorrhoids in the United States
- According to IMS, over 25 million units of topical combination prescription products for are sold in the US

Although there are numerous prescription and over-the-counter (OTC) products commonly used to treat hemorrhoids, none currently possess safety and efficacy data generated from rigorously-conducted clinical trials. Citius believes its proprietary formulation will become an important treatment option for physicians who want to provide their patients with a therapy that has demonstrated safety and efficacy in treating hemorrhoids, an uncomfortable and often recurring condition.
If Citius receives FDA approval for its topical halobetasol-lidocaine combination formulation for the treatment of hemorrhoids, it may qualify for three years of market exclusivity for its dosage strength and formulation. In that case, Halo-Lido may be the only product on the market proven to be safe and effective for the treatment of hemorrhoids.
NCi -MSC (Stem Cells)
Next-generation engineered stem cell platform of induced mesenchymal stem cells (i-MSCs)
Citius is developing a unique, proprietary stem cell platform for the treatment of respiratory conditions associated with acute inflammation, with an initial indication in the treatment of acute respiratory distress syndrome (ARDS).
Citius’ i-MSCs are derived from iPSCs originating from a qualified single-donor dermal fibroblast, resulting in one homogeneous, validated source for all future cells. A patented synthetic, non-immunogenic mRNA (non-viral) high efficiency cell reprogramming technique is applied and expanded under cGMP guidelines to create a clonal iPSC Master Cell Bank. The i-MSCs produced from this clonal technique are differentiated from human donor-derived MSCs (bone marrow, placenta, umbilical cord, adipose tissue, or dental pulp) by providing genetic homogeneity. In vitro studies showed that Citius’s i-MSCs exhibit superior potency and high cell viability. Moreover, i-MSCs secrete immunomodulatory proteins that may reduce or prevent pulmonary symptoms associated with ARDS. Citius believes that the characteristics of its single-donor i-MSCs support clonal production at scale with consistent quality and greater potency, and may offer patients and clinicians a promising treatment option for ARDS.
Program Highlights
- Novel stem cell therapy for the treatment of acute inflammatory respiratory disorders including acute respiratory distress syndrome (ARDS)
- i-MSCs derived from induced pluripotent stem cell reprogrammed using proprietary mRNA process
- No FDA-approved treatment for ARDS exists today
- Preclinical activities are underway
Stem cells are an exciting new area of focus as potential therapies for ARDS, and are currently the subject of ongoing research efforts worldwide. Citius believes mesenchymal stem/stromal cells (MSCs) offer considerable promise for ARDS. Several donor-derived MSC therapies under investigation have demonstrated that MSCs may reduce inflammation, enhance clearance of pathogens and stimulate tissue repair in the lungs. MSCs have also been shown to restore endothelial and epithelial barrier integrity, enhance the clearing of fluid from the lungs, and may exhibit antimicrobial properties.
Citius is exploring the potential of its i-MSCs to overcome the limitations of MSCs derived from adult donors. Positive interim results from a proof-of-concept study demonstrate a marked improvement in i-MSC-treated animals over control animals in key clinical parameters. Learn more….
Mino-Wrap
Bioabsorbable extended-release antimicrobial wrap for the prevention of breast tissue expander infections. 
Mino-Wrap (CITI-101) is a malleable, bio-absorbable film impregnated with minocycline and rifampin. It is a novel therapeutic designed to significantly reduce infections associated with the use of breast tissue expanders (TE) used in patients that elect to undergo reconstructive breast surgery.
Program Highlights
- Potential to be first and only FDA-approved product to prevent infections associated with post mastectomy breast implants
- Currently in pre-clinical development
- Development in partnership with The University of Texas MD Anderson Cancer Center and support from medical thought leaders
How it Works
Mino-Wrap is designed to allow the temporary tissue expander used in breast reconstruction surgeries to be inflated without any restrictions, and to aid in the prevention of infection and biofilm formation on the implant over longer durations than current practice. It is placed over or wrapped around the TE in the surgical pocket as a solid film. It swells and liquefies in situ for a specified period of time to provide extended protection against infection from the most likely pathogens.CATALYSTS
- Diversified pipeline of potential first-in-class products with multiple near-term staged catalysts
- Attractive multi-billion-dollar opportunities in adjunctive cancer care, infectious disease and gastrointestinal disease
- Strong research partnerships to advance the pipeline
- Robust balance sheet to support pipeline development and invest in long-term growth
- Seasoned leadership with successful execution-focused track record
Citius is guided by three principles in our mission to deliver best-in-class therapies for patients with critical unmet needs.
- Advance therapies with unique commercial advantages
- Invest in assets with differentiated upside potential
- Create long-term sustainable value for shareholder
TRIALS
Mino-Lok Therapy (MLT) for the Treatment of CRBSI/CLABSI Learn more
Antimicrobial Catheter Lock Solution for the Treatment of Central Line Associated Bloodstream Infection (CLABSI) Learn more
Expanded Access: Mino-Lok Therapy (MLT) for the Treatment of CRBSI/CLABSI (MLK) Learn more
Publications

Successful Salvage of Central Venous Catheters in Patients with Catheter-Related or Central Line-Associated Bloodstream Infections by Using a Catheter Lock Solution Consisting of Minocycline, EDTA, and 25% Ethanol

Treating CLABSI: A Clinical and Economic Challenge

Unnecessary Removal of Central Venous Catheters in Cancer Patients with Bloodstream Infections: Impact on Symptom Burden

Novel Induced-Mesenchymal Stem Cells (i-MSCs) Attenuate Severity of ARDS in Septic Sheep
Mino-Lok®
Phase 3 TrialBased on Phase 2b results, Citius Pharmaceuticals believes that Mino-Lok® is highly effective in salvaging infected indwelling catheters and is well-tolerated, making Mino-Lok® therapy an attractive alternative to removing and replacing a CVC.
- To evaluate the efficacy of Mino-Lok® along with standard of care (SOC) systemic antibiotics for salvaging the central venous catheter (CVC) in subjects with catheter-related or central line-associated bloodstream infection (CRBSI/CLABSI)
- To evaluate the safety of Mino-Lok® in subjects with CRBSI/CLABSI
Phase 2b TrialCitius Pharmaceuticals completed a Phase 2b study in December 2014. There were 90 patients in the study, with 30 patients in the active arm and 60 patients in a matched cohort for comparison. All patients were receiving treatment at The University of Texas MD Anderson Cancer Center for hematologic or solid tumor cancers.
Outstanding Comparative ResultsMino-Lok® salvaged 100% of CVCs, helping to cure all of the bacterias with no serious adverse events, compared to an 18% serious adverse event rate in the matched cohort where patients had the infected CVCs removed and replaced with a new CVC. The published manuscript can be downloaded here.

* One polymicrobial patient had a Gram+ and a Gram– organism cultured
** Six patients had more than one complication
*** All 3 CVCs were removed within 1 month.
Halo-Lido
Phase 2a Study*
In a randomized, double-blind study, 210 patients with Grade I and II hemorrhoids were treated twice daily for 14 days. Patients received either a placebo or one of six active drug treatments, with two concentrations each of hydrocortisone, lidocaine, or a hydrocortisone-lidocaine combination. Patients kept a diary of their symptoms.
Additionally, there were four physician assessments, during which patients were evaluated on the Global Score of Disease Severity (GSDS) scale as well as on the individual signs and symptoms of hemorrhoids, such as bleeding, pruritus, overall pain and discomfort, and time to the onset of symptom relief.
Level Improvement Global Severity

Within the first few days of treatment, the highest concentration of the hydrocortisone lidocaine combination was directionally superior to the placebo as measured by the number of subjects experiencing a minimum of two levels improvement from baseline according to the GSDS scale. This study was not powered to obtain statistical significance; however, the data suggest that the combination product may also perform better than hydrocortisone or lidocaine alone.
Pruritus (Severe Itching)

The hydrocortisone-lidocaine combination seemed to achieve 88.9% greater relief of pruritus at Day 2 as compared to any of its components alone.
Pain and Discomfort

The hydrocortisone-lidocaine combination seemed to achieve 85.7% greater relief of pain and discomfort at Day 2 as compared to any of its components alone.
* Study was not powered to show statistical significance; its purpose was to inform future study designs.
CITI-001 (Hydro-Lido) was the first steroid-anesthetic combination product tested in a clinical trial for symptomatic relief of hemorrhoids. The new formulation, CITI-002, combines lidocaine with the higher-potency corticosteroid for symptomatic relief of the pain and discomfort of hemorrhoids. This change should improve the efficacy further while also providing faster onset of relief. While not used in combination in currently marketed products, the proposed corticosteroid is included as an FDA-approved topical product to treat a variety of dermatological disorders.
Stem Cell Platform
Citius is conducting a proof-of-concept study to test the safety and efficacy of a novel potent iPSC induced MSCs ( i-MSCs) in a clinically relevant sheep model of sepsis-induced ARDS. Interim results show that animals receiving i-MSCs demonstrated clear improvement over control animals with improved oxygenation, less systemic shock and reduced lung vascular injury.
While the interim results of the study have shown that use of i-MSCs is safe and effective in ameliorating severity of sepsis-induced acute lung injury, further studies of increasing sample sizes are warranted. Data from this sheep ARDS study will inform the design and dosing of planned future human clinical trials in ARDS using these i-MSCs.
Interim results of i-MSCs in the proof-of-concept study:
- Displayed characteristics of donor MSCs by differentiating to adipocytes, osteoblasts and chondrocytes
- Population doubling was ~4-fold higher vs. that of bone marrow-derived MSCs
- Improved oxygenation and prevented onset of ARDS
- Reduced pulmonary microvascular hyperpermeability to water and protein and ameliorated severity of pulmonary edema
- Reduced fluid requirement
- Reduced vasopressor requirement to maintain arterial blood pressure
- Significantly improved bacterial clearance
- Had no hemodynamic adverse effects
NEWS
MANAGEMENT
Leonard MazurExecutive Chairman, Director
Mr. Mazur is an accomplished entrepreneur and pharmaceutical industry executive with notable accomplishments in founding and building multiple healthcare companies, and creating value and returns for investors. Mr. Mazur was the Chairman of Leonard-Meron Biosciences, Inc. prior to its merger with Citius in March 2016. He is also the cofounder and Vice Chairman of Akrimax Pharmaceuticals, LLC, a privately held pharmaceutical company specializing in producing cardiovascular and general pharmaceutical products. Akrimax was founded in September 2008, and has successfully launched prescription drugs while acquiring drugs from major pharmaceutical companies. From 2005 to 2012, Mr. Mazur co-founded and served as the Chief Operating Officer of Triax Pharmaceuticals LLC, a specialty pharmaceutical company producing prescription dermatological drugs. Earlier, he was the founder and Chief Executive Officer of Genesis Pharmaceuticals, Inc., a dermatological products company that marketed its products through dermatologists’ offices and co-promoted products for major pharmaceutical companies. In 2003, Mr. Mazur successfully sold Genesis to Pierre Fabre, a leading pharmaceutical company. Mr. Mazur has extensive sales, marketing and business development experience from previous tenures at Medicis Pharmaceutical Corporation, ICN Pharmaceuticals, Inc., Knoll Pharma (a division of BASF), and Cooper Laboratories, Inc.
Mr. Mazur is a member of the Board of Trustees of Manor College, and is a recipient of the Ellis Island Medal of Honor. Mr. Mazur received both his BA and MBA from Temple University, and served in the U.S. Marine Corps Reserves.
Myron HolubiakPresident and Chief Executive Officer, Director
Mr. Holubiak has extensive experience in managing and leading both large and emerging pharmaceutical and life sciences companies. Mr. Holubiak was co-founder, director and CEO of Leonard-Meron Biosciences, Inc. prior to its merger with Citius in March 2016. From 1998 to 2001, Mr. Holubiak served as President of Roche Laboratories, Inc., a premier multinational research-based pharmaceutical company. As President of Roche, Mr. Holubiak helped transform Roche Labs into a leading antibiotic and biotechnology company. During his 19-year tenure at Roche Labs, Mr. Holubiak also held multiple sales and marketing roles. Prior to Roche, Mr. Holubiak founded Emron, Inc., a health economics and managed care consulting company, and helped establish the Academy of Managed Care Pharmacy (AMCP). From 2012 to 2016, Mr. Holubiak served as Chairman of the Board of Bioscrip, Inc., a national home infusion company. Since 2010, Mr. Holubiak has served as a member of the Board of Directors of Assembly Biosciences, Inc. and its predecessor, Ventrus Biosciences, Inc., and is a trustee of the Academy of Managed Care Pharmacy Foundation.
Mr. Holubiak received a BS in molecular biology and biophysics from the University of Pittsburgh. He received advanced business training from Harvard Business School and the University of London, as well as advanced training in health economics from the University of York’s Centre for Health Economics.
Jaime BartushakChief Financial Officer
Mr. Bartushak is an experienced finance and operations professional for early-stage pharmaceutical companies, and has over 20 years of corporate finance, business development, M&A, restructuring, capital formation, and strategic planning expertise. Mr. Bartushak is a founder of Leonard-Meron Biosciences, and, as CFO, was instrumental in obtaining initial investment capital for its start-up in 2014. Earlier, Mr. Bartushak helped lead the sale of PreCision Dermatology, Inc. to Valeant Pharmaceuticals International, Inc., and before that, he led the financial efforts for the successful sale of Triax Pharmaceuticals to PreCision Dermatology.
Mr. Bartushak holds a Master of Science and BS from the New Jersey Institute of Technology.
Myron S. Czuczman, MDChief Medical Officer and EVP
Dr. Czuczman is an experienced physician-scientist, academic oncologist, and pharma executive with decades of experience in strategic design, implementation, and oversight for the global development of novel therapeutics for hematologic malignancies. Dr. Czuczman joined Citius from Celgene where he was Vice President, Global Clinical Research and Development, Therapeutic Area Head of Lymphoma/CLL. In this role, Dr. Czuczman managed a global team of physicians and scientists responsible for cross-functional development of compounds from proof-of-principle to worldwide registration. Prior to his career in pharma, Dr. Czuczman practiced medicine for over two decades at Roswell Park Cancer Institute, an NCI-designated comprehensive cancer center in Buffalo, NY, where he served as chief of the Lymphoma/Myeloma Service and head of the Lymphoma Translational Research Laboratory. In addition to his extensive publications record, membership and leadership roles on national and international research organizations, and consulting and advisory to dozens of pharma companies, Dr. Czuczman also attained the positions of tenured Professor of Medicine at the State University of New York at Buffalo School of Medicine and Biomedical Sciences and Professor of Oncology at Roswell Park Comprehensive Cancer Center.
Dr. Czuczman received his medical degree from the Pennsylvania State University College of Medicine after graduating magna cum laude in biochemistry from the University of Pittsburgh. He completed his Internal Medicine residency training at Weill Cornell North Shore University/MSKCC Program, followed by Medical Oncology/Hematology fellowship training at Memorial Sloan-Kettering Cancer Center in New York City.
Gary F. TalaricoEVP, Operations
Mr. Talarico has served as EVP, Operations since March 2016. Mr. Talarico has successfully built and led all commercial activities for a number of start-up companies. Most recently, he was a founder, partner and Executive Vice President of Leonard-Meron Biosciences, Inc.; he was instrumental in acquiring its lead product. Previously, Mr. Talarico served as Senior Vice President of Triax Pharmaceuticals, from its founding to the sale of its assets. Mr. Talarico was a founder and Executive Vice President of Sales and Marketing for Reliant Pharmaceuticals, LLC; Reliant was later sold to GlaxoSmithKline plc. Before Reliant, he was Executive Vice President of Business Development for Ventiv Health. His earlier experience included tenures as Vice President of Sales for Medicis Pharmaceutical Corporation at its start-up, and Director of Sales at ICN Pharmaceuticals, Inc. Mr. Talarico is a graduate of Lewis University.
Jay WadekarSVP, Business Strategy
Mr. Wadekar has been associated with Citius since its inception. Prior to Citius, he lead the clinical program at Ischemix, Inc., a company developing novel therapies for cardiovascular conditions. Mr. Wadekar has more than thirty years of experience in areas of finance, corporate strategy, sales and senior leadership in the healthcare field. Mr. Wadekar has held numerous executive level positions throughout his career in biotechnology and pharmaceutical industries including Chairman and CEO of Able Laboratories, Inc. Most recently he served as a strategic advisor to Camber Pharmaceuticals, Inc. where he was instrumental in building the executive team and establishing Camber’s Sales Operations systems.
Alan Lader, PhDVP, Clinical Operations
Dr. Lader has served as VP, Clinical Operations since March 2016. Dr. Lader has over 25 years of experience in medical research. Prior to joining Citius, Dr. Lader was the Director of Clinical Operations for Ischemix, Inc. Dr. Lader was an Instructor in Medicine at Harvard Medical School and Brigham and Women’s Hospital, where he taught Integrated Human Physiology, and was Principal Investigator for NIH-funded studies in mechanisms of lung cancer metastasis. Dr. Lader has authored over 20 publications in peer-reviewed journals, and has presented more than 20 abstracts at scientific meetings. He received his PhD from the University of South Carolina School of Medicine. He received an MS degree from Rensselaer Polytechnic Institute in Biomedical Engineering and a BS degree in Bioengineering from Syracuse University.
Ilanit AllenVP, Investor Relations
Ms. Allen has more than 20 years of experience in corporate communications, investor relations, strategy and investment banking. Since 2014, Ms. Allen has provided investor relations counsel to more than two dozen private and public life science companies. Previously, she advised executives across a broad spectrum of industries and growth stages, including technology startups and Fortune 500 financial institutions. Ilanit began her career as an investment banking analyst at SG Cowen with a focus on mergers and acquisitions. Ms. Allen holds an MBA from Harvard Business School, a Bachelor of Science degree in Finance from The Wharton School, and a Bachelor of Arts degree in International Relations from the University of Pennsylvania.
Sincerely,
The Viral Stocks Team
DISCLAIMER
THIS WEBSITE/NEWSLETTER IS A WHOLLY OWNED SUBSIDIARY OF GLOBAL COIN MEDIA, LLC. HEREIN REFERRED TO AS GCM, LLC. WE ARE A NEVADA COMPANY.
OUR REPORTS/RELEASES ARE A COMMERCIAL ADVERTISEMENT AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. WE HAVE BEEN COMPENSATED A FEE OF FIFTEEN THOUSAND USD BY A THIRD PARTY, LEGENDS MEDIA, LLC FOR A ONE DAY CTXR AWARENESS CAMPAIGN.WE HAVE PREVIOUSLY BEEN COMPENSATED A FEE OF FIFTEEN THOUSAND USD FOR A TWO DAY CTXR AWARENESS CAMPAIGN BY A THIRD PARTY, LEGENDS MEDIA, LLC. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR SITE OR OUR EMAIL/BLOG LIST AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE.PLEASE NOTE WELL: GCMLLC AND ITS EMPLOYEES ARE NOT A REGISTERED INVESTMENT ADVISOR, BROKER DEALER OR A MEMBER OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER.RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE VIEWING OR USING YOU AGREE TO HOLD GCM, LLC, ITS OPERATORS OWNERS AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES WHICH WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. GCMLLC ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED, OR IS AVAILABLE FROM PUBLIC SOURCES AND GCM, LLCMAKES NO REPRESENTATIONS, WARRANTIES OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD GCM, LLC. STRONGLY URGES YOU CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D.GCM, LLC IS COMPLIANT WITH THE CAN SPAM ACT OF 2003. GCM, LLCDOES NOT OFFER SUCH ADVICE OR ANALYSIS, AND GCM, LLCFURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AND EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD LOOKING STATEMENTS. FORWARD LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTE; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS.IN PREPARING THIS PUBLICATION,GCM, LLCHAS RELIED UPON INFORMATION SUPPLIED BY ITS CUSTOMERS, PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CANNOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED IN THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, GCM, LLCAND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. GCM, LLCIS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS GCM, LLCRESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM OR ITS STRUCTURE. GCM, LLC IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION OR FINRA.













