OUR NEW PROFILE IS: (NASDAQ: BTCY)
BTCY has shown triple-digit, quarter-over-quarter, year-over-year growth since day 1 (10 consecutive quarters)
Reported Revenue of $1.93 million, an increase of 93% year-over-year and sequentially 7% above the $1.8- million reported in Q2FY22.
BTCY has been rated by Maxim Group, Noble Financial, and Northland Securities in the past year, with price targets of $6 & $7
Biotricity Receives FDA 510(k) Clearance for its Biotres Cardiac Monitoring Device
Biotricity Expands Distribution of New Bioheart Device on Amazon.com
___________________________
Hello Everyone,
Turn your attention to Nasdaq: BTCY.
BTCY was trading on the OTC just a few months back. It has recently completed it’s up-listing to the Nasdaq, an accomplishment that many talk about and very few are actually able to execute on.
Biotricity named to Fast Company’s annual list of the World’s 50 Most Innovative Companies for 2022
*****Breaking News*****
Biotricity to Launch Commercial Sales of Its Disruptive Cardiac Monitoring Device, Biotres in April 2022
- Company reports pre-order sales from existing customers of its wireless wearable device for early detection of cardiac arrhythmias
REDWOOD CITY, CA / ACCESSWIRE / March 22, 2022 /Biotricity, Inc.(NASDAQ:BTCY) (“Biotricity” or the “Company”), a medical diagnostic and consumer healthcare technology company, will officially launch the commercial sales of its FDA cleared, wireless wearable cardiac monitoring device, Biotres, in April 2022. The product has been available for pre-orders to physicians, medical offices, hospitals and individual use since late-February 2022.
Biotres is the Company’s revolutionary technology that represents the future of Remote Patient Monitoring and the delivery of real-time diagnostic data. It serves as a three-lead device designed to continuously record electrocardiogram (ECG) data for early detection of cardiac arrhythmias, disrupting the conventional one-lead patch Holter monitor which takes longer analysis time and diagnosis time.
Biotres received FDA 510(k) clearance in January 2022, opening up a new market and expanding Biotricity’s total addressable market to $5.7 Billion. Since taking pre-orders last month, the Company has experienced strong demand for Biotres from existing customers. The Company has plans to roll out additional complementary products throughout 2022, following the same approach as Biotres. Each solution will grow the company’s TAM, creating new revenue streams for existing customers while expanding its customer base.
Dr. Waqaas Al-Siddiq, Biotricity Founder and CEO, commented, “After months of anticipation, our Biotres product is finally available for sale. Judging by the amount of pre-order interest we’ve seen since February, Biotres can provide millions of individuals with an early detection method to easily and efficiently record cardiac arrhythmias. We are optimally positioned to penetrate a $2 billion-dollar holter market that needs a faster and more efficient approach, critical to improving patient lives.”
You can purchase biotres through the following link: https://www.biotricity.com/biotres/
Management to host investor call on Tuesday February 15th at 4:15 pm ET
- 93% YOY Revenue Gains to $1.93 Million
- Revenue Outpaces SG&A Increase by 2.2x
- Cash Position of $16.8 Million at Quarter End Is Highest Ever
- Continues to Anticipate Full-Year Triple-Digit Revenue Growth
FEBRUARY 14, 2022 | BY BIOTRICITY
Biotricity Receives FDA 510(k) Clearance for its Biotres Cardiac Monitoring Device
Biotres is designed to continuously record ECG data for early detection of cardiac arrhythmias
The Biotres addresses the $2 Billion-dollar holter market
REDWOOD CITY, CA / ACCESSWIRE / January 24, 2022 / Biotricity, Inc.(NASDAQ:BTCY) (“Biotricity” or the “Company”), a medical diagnostic and consumer healthcare technology company, today reported it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Biotres Cardiac Monitoring Device, a three lead device for ECG and arrhythmia monitoring that is intended for lower risk patients.
Dr. Waqaas Al-Siddiq, Biotricity Founder and CEO commented, “We are extremely proud of this accomplishment and its potential to reshape the future of cardiac prevention and monitoring with this novel platform. With Biotres receiving 510(k) clearance, we now have another option for providing a secondary product for doctors and hospitals to meet their patients’ cardiac monitoring needs. Coupled with the recent successful non-dilutive financing, we believe we are well capitalized to execute the expansion of our commercialization efforts.”
“Biotres, is a holter for low-risk patients and is designed for comfort for long-term wear,” he added. “Cardiac disease remains a chronic issue, requiring persistent intervention, monitoring and management and we believe Biotres may provide a critical tool for improving patient lives and reducing costs.”
Designed to address the current limitations of existing holter monitors technologies, Biotres offers the following feature set:
- 3 Channel Recording – A wearable holter patch device that can provide continuous 3 channel recording of ECG (heart) data. All other known holter patch devices are 1 channel or 2 channels.
- Rechargeable Battery – It can be worn continuously for 48 hours, in connectivity mode, before needing to be charged for 1 hour, enabling continuous data collection for extended periods of time without any intervention, a distinction not possible with traditional holter patch solutions.
- Wireless Connectivity – Utilizes Bluetooth technology to offload data, reducing the time for diagnoses. Current holter patch solutions can take up to a week before diagnoses are available due to manual data downloading and a lack of connectivity.
- User-Friendly Design – Easy to understand and comfortable to wear during regular day-to-day activities.
- Modular Design – Designed with the flexibility to support our strategy of adding future features and functionality to the Biotres platform.
- Unique Business Model – the design of Biotres enables providers to bill directly, creating a revenue stream with a simplified workflow for providers while reducing risk and diagnostic turnaround time for patients.
The Biotres not only expands our product portfolio, it opens up a new market and increases Biotricity’s Total Addressable Market from $1B to $5.7 Billion and will be widely available for sale starting April 1, 2022.
Biotricity Expands Distribution of New Bioheart Device on Amazon.com
New Heart Monitoring Solution Delivers Personalized Heart Health Insights Directly to Your Smartphone
Now Easy to Buy and Use: No Prescription Needed for Advanced Heart and Lifestyle Solution. 
REDWOOD CITY, CA / ACCESSWIRE / December 28, 2021 /Biotricity Inc.(NASDAQ:BTCY) a modern medical technology company delivering innovative, remote biometric monitoring solutions, today announced it has expanded the distribution of its new Bioheart heart monitor system, a direct-to-consumer device that offers the same continuous heart monitoring technology used by physicians, on Amazon.com.
Last month the company launched commercial sales of its Bioheart device. To improve availability and expand outreach, Biotricity has now listed it for purchase on Amazon.com at: Bioheart on Amazon for $199 with a special introductory price of $149 for a limited time. You can view the Bioheart heart monitor in use here.
“We are aiming to transform the cardiac health marketplace and believe the Bioheart system will play an important role in that endeavor,” said Dr. Waqaas Al-Siddiq, Biotricity Founder and CEO, “The Bioheart device is an important component of our full spectrum, ‘virtual cardiac clinic’ of mobile cardiac care and lifestyle solutions. It will be fully integrated with a growing number of cardiology practices through our cloud-based ‘Biosphere’ ecosystem that we plan to fully roll out in 2022, allowing physicians to get a more holistic view of a patient’s health.
“The ability to offer Bioheart on Amazon expands the availability for the public to easily access the benefits of this exciting technology,” Dr. Waqaas added.
The launch of Bioheart opens a new market and revenue stream for Biotricity while expanding the company’s total addressable market by $1.24B. Heart conditions are intermittent, requiring long-term data collection for effective insights and lifestyle management. With continuous monitoring, Bioheart reinvents personal heart management with retrospective snapshots and long-term data collection.
Introducing a groundbreaking new capability, users can collect data for hours, days, weeks, months, and even years! They can create snapshots of rhythm data by reviewing past data and marking it for personal records. These are first-of-its-kind features that will help individuals look back at their data for a richer understanding of their lifestyle impact and heart health.
Bioheart features include:
- Built-in dry contacts for data collection
- Advanced data collection – three different heart views
- 24/7 continuous rhythm recording
- 48-hour battery life
- Personalized analytics
- Bluetooth connected
With the Bioheart smartphone app, users can:
- View electrical heart rhythm live on the app
- View data on advanced heart metrics such as electrical heart rhythm, heart rate and heart rate variability
- View historical heart rate ranges
- Create a heart rhythm diary and save snapshots of heart activity
- Easily create retrospective snapshots, a first-of-its kind capability
- Easily export and share heart data with a physician
Bioheart incorporates the Company’s proprietary advanced heart technology, combined with powerful analytics and continuous rhythm recording, to help individuals understand and improve their heart health. Individuals can track their heart statistics 24/7 with three different views of the heart, which can be streamed live on the Bioheart smartphone app. Bioheart is the most accurate heart monitor available without a prescription.
Bioheart retails for $199 and is available at a special price of $149, for a limited time, on Amazon or www.bioheart.com. The app is available on Apple’s App Store and on Google Play.
Biotricity Delivers Bioheart, A Groundbreaking Consumer Heart Monitor
Continuous monitoring device with advanced features and analytics to deliver personalized insights directly to the smartphone
REDWOOD CITY, Calif., November 10, 2021–(BUSINESS WIRE)–Biotricity, Inc.(NASDAQ:BTCY), a modern medical technology company focused on delivering innovative, remote biometric monitoring solutions, today announced the availability of Bioheart, a direct-to-consumer heart monitor that offers the same continuous heart monitoring technology used by physicians. Showcased in prototype form at CES 2020, Bioheart has shipped to preorder customers and is now available for purchase at www.bioheart.com.
The launch of Bioheart opens a new market and revenue stream for Biotricity while expanding the company’s total addressable market by $1.24B, the global heart rate monitoring market. “Launching Bioheart and expanding into this new market is an important step for Biotricity and our ultimate goal of building a complete cardiac ecosystem to service individuals throughout their heart health journey,” said Dr. Waqaas Al-Siddiq, Founder and CEO of Biotricity.
Heart conditions are intermittent, requiring long term data collection for effective insights. With continuous monitoring, Bioheart reinvents personal heart management with retrospective snapshots. Introducing a groundbreaking new capability, users can create snapshots of rhythm data by reviewing past data and marking it for personal records. This is a first-of-its-kind feature that will help individuals look back at their data for a richer understanding of their lifestyle impact and heart health.
Bioheart incorporates the Company’s proprietary advanced heart technology, combined with powerful analytics and continuous rhythm monitoring, to help individuals understand and improve their heart health. It provides 24/7 electrical heart rhythm monitoring and recording with three different views of the heart, which can be streamed live on the Bioheart smartphone app. Bioheart is the most accurate heart monitor available without a prescription.
“Bioheart combines unparalleled accuracy in a heart monitoring device with continuous data, advanced heart health insights, and powerful features tailored to individuals at risk for heart disease or who want precise data for performance tracking, optimization and preventative cardiac wellness,” said Dr. Al-Siddiq. “Bioheart furthers Biotricity’s mission of developing revolutionary healthcare solutions that help engage and empower individuals to take control of their health, starting with the deadliest chronic disease and one of healthcare’s largest cost drivers: cardiac disease.”
Heart condition signs are typically intermittent, and most heart monitoring devices only capture short periods of heart activity. These devices have limited capabilities and do not provide individuals with the heart data or tools needed to support preventative care and effective lifestyle improvements. With Bioheart’s continuous monitoring and long-term data collection approach, users can analyze heart data from previous months, well after the initial recording, to understand changes and long-term trends.
“Bioheart has the potential to make an immediate, life-changing impact for those who use it,” added Dr. Al-Siddiq. “As a direct manufacturer, we’re thrilled to have been able to accelerate our market delivery to the general market in less than a year, ahead of schedule and in time for the busy holiday gifting season.”
Bioheart retails for $199 and is available for purchase here or at www.bioheart.com. The app is available on Apple’s App Store and on Google Play.
FLAGHSHIP PRODUCT
BTCY is Targeting a Growing and Underserved Market with the Bioflux.
The company’s first and primary solution, Bioflux, addresses the growing and underserved Mobile Cardiac Telemetry (MCT) market.
Bioflux is a high-precision, single-unit MCT device providing real-time monitoring and transmission of ECG information from ambulatory patients. This entire system is a complete solution for remote cardiac monitoring that merges seamlessly with physicians’ existing platforms and workflows to better detect, diagnose, and monitor.
For medical practitioners, this solution looks to be technologically superior and more financially attractive too. Clinicians designed the Bioflux online portal for clinicians. After all, the prime features of the product were built after speaking with numerous healthcare professionals and seeing what their most requested features were.
They track over 100 billion heartbeats a year, monitored in real-time by their certified cardiac technicians with unmatched reporting capabilities.
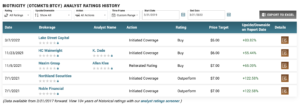
ANALYST RATINGS

Biotricity (OTCMKTS:BTCY) Analyst Ratings Frequently Asked Questions
WHAT IS BIOTRICITY’S CONSENSUS RATING AND PRICE TARGET?
According to the issued ratings of 5 analysts in the last year, the consensus rating for Biotricity stock is Buy based on the current 5 buy ratings for BTCY. The average twelve-month price target for Biotricity is $6.40 with a high price target of $7.00 and a low price target of $5.00. Learn more on BTCY’s analyst rating history.
DO WALL STREET ANALYSTS LIKE BIOTRICITY MORE THAN ITS COMPETITORS?
Analysts like Biotricity stock more than the stock of other Medical companies. The consensus rating score for Biotricity is 3.00 while the average consensus rating score for medical companies is 2.73. Learn more on how BTCY compares to other companies.
DO MARKETBEAT USERS LIKE BIOTRICITY MORE THAN ITS COMPETITORS?
MarketBeat users like Biotricity stock less than the stock of other Medical companies. 66.67% of MarketBeat users gave Biotricity an outperform vote while medical companies recieve an average of 67.45% outperform votes by MarketBeat users.
DOES BIOTRICITY’S STOCK PRICE HAVE MUCH UPSIDE?
According to analysts, Biotricity’s stock has a predicted upside of 87.03% based on their 12-month price targets.
WHAT ANALYSTS COVER BIOTRICITY?
Biotricity has been rated by Lake Street Capital in the past 90 days.
(sourced from MarketBeat)
*****RECENT NEWS*****
Biotricity, a Leader in Remote Cardiac Health Monitoring Solutions, Issues Company Roadmap in 2021 Shareholder Letter
The Company believes that it is “well-positioned for 2022.”
Letter highlights milestones, products roadmap, growth strategy and 2022 goals
REDWOOD CITY, CA / ACCESSWIRE / December 7, 2021 / Biotricity, Inc.(NASDAQ:BTCY) (“Biotricity” or the “Company”), a medical diagnostic and consumer healthcare technology company, has released its December 2021 CEO Shareholder Letter, which discusses recent Company milestones achieved including its financial performance, commercialization of its new Biokit, and other state-of-the-art remote cardiac monitoring devices and software apps in development.
Highlights from the shareholder letter include:
- Recent uplisting to Nasdaq that the Company believes will help further attract a larger pool of sophisticated institutional investors, and a potential increase in liquidity and visibility
- Commercialization of the personal medical 3-devices kit, Biokit, for integration into the Biotricity ecosystem
- Record quarterly revenue of $1.81 million for its 2022 fiscal year’s second quarter ended September 30, 2021, up 143% year-over-year – its tenth consecutive quarter of triple-digit year-over-year growth
- The product roadmap for Bioflux®, Biotres, Biocare® Telemed, Biokit, and Bioheart
- The Company’s 2022 goals including launching new state-of-the-art cardiac monitoring solutions and software services.
Dr. Waqaas Al-Siddiq, Founder and CEO of Biotricity commented, “We had a historic second quarter that saw revenue increase 143% YoY, the strengthening of our balance sheet, and uplisting to Nasdaq which has boosted our exposure to investors worldwide. We’ve made great strides in each vertical we targeted over the year and are well-positioned to continue the execution of our overall growth plan for 2022. We seek to take telemedicine to a new, next-generation level of cost-effectiveness as well as disrupt the cardiac care landscape by reducing patient care costs with improved patient outcomes.”
The Biotricity December 2021 Shareholder Letter can be viewed at https://www.biotricity.com/wp-content/uploads/2016/09/Biotricity_Letter-To-Shareholders-Dec21.pdf
Biotricity named to Fast Company’s annual list of the World’s 50 Most Innovative Companies for 2022
Leader in early-detection cardiac diagnostics monitoring solutions joins the ranks of Canva, Microsoft, SpaceX, and more
MARCH 08, 2022 | BY BIOTRICITY
Biotricity Unveils “Biokit” with 3 Personal Medical Devices to Expand into Cardiac Disease Management
Company Expects New Personal Medical Device Kit to be available to customer by early 2022
REDWOOD CITY, CA / ACCESSWIRE / September 14, 2021 /Biotricity Inc.(NASDAQ:BTCY), a medical diagnostic and consumer healthcare technology company, today announced that it has developed a personal medical device kit, Biokit, a bundled home-use set comprised of a digital thermometer, a pulse oximeter, and a blood pressure cuff. All three devices are FDA cleared, wireless capable and integrate into the Biotricity ecosystem, a platform for interconnectivity. This is the next step in the evolution of the Bioflux solution, where other available tools are fully integrated to create a complete solution for remote cardiac monitoring.

“We created the Biokit to strengthen our long-term vision of supporting diagnosed cardiac patients throughout their cardiac health journey,” Waqaas Al Siddiq, Biotricity CEO explained. “Biokit is a natural extension of our Bioflux cardiac-diagnostic offering. For patients with cardiac disease, a personal medical device kit is ideal for long term monitoring. After speaking with both patients and physicians, it was clear that a product like this was an immediate need. I am pleased with how quickly we have been able to develop a solution to fulfill this customer feedback, which is a testament to the team here. The Biokit will expand our total addressable market with a tailored offering for the $18.4 billion-dollar North American portable medical device market1. With Bioflux and Biokit combined, Biotricity will soon offer a comprehensive cardiac diagnostics and management product line for effective, accurate and economic long-term disease management.”
The Biokit is a wirelessly connected personal medical device kit that was developed to address the challenges with existing home-based medical devices. It includes the following devices, all of which integrate with the Biotricity ecosystem and provide near real-time data to both the physician and the patient through the web and an App:
- Biokit BP – A wireless digital blood pressure cuff enabling patients to collect BP data.
- Biokit TP – A wireless digital thermometer enabling patients to collect temperature data.
- Biokit 02 – A wireless digital pulse oximeter enabling patients to collect blood-oxygen data.
- Biotricity Ecosystem Integration – All devices within the Biokit are seamlessly integrated into the Biotricity ecosystem, enabling patients to track and store their data. Most importantly, this data is also stored and available for physician interim review and for follow up visits.
CHART
NEWS
Management Team
Dr. Waqaas Al-Siddiq, Phd
Chairman, CEO & Founder
Waqaas, the founder of Biotricity, is a serial entrepreneur, a former investment advisor and an expert in wireless communication technology. Academically, he was distinguished for his various innovative designs in digital, analog, embedded, and micro-electro-mechanical products. His work was published in various conferences such as IEEE and the National Communication Council.
Waqaas has held several high-level design positions in IBM, AMD, and Intel. His achievements have been numerous in both the technical and academic world. Coupled with this, Waqaas has vast experience in leading various groups through his board experience and executive roles within start-ups, mid-sized companies, and non-profits.
Waqaas has a dual Bachelor’s degree in Computer Engineering and Economics, a Master’s in Computer Engineering from Rochester Institute of Technology, and a Master’s in Business Administration from Henley Business School. He also holds a Doctorate in Business with a specialty in Transformative Innovations and Billion Dollar Markets.
Norman Betts, Phd
Independent Board Member
Norman sits on the Boards of Directors of the Bank of Canada, Tembec Inc., New Brunswick Power Corporation, 49 North Resources Inc., Adex Mining Inc., Tanzanian Royalty Exploration Inc. and the University of New Brunswick Pension Plan for Academic Employees.
Norman is an Associate Professor in the Faculty of Business Administration at the University of New Brunswick in Fredericton. He is accomplished in the field of accounting and finance, with a mix of academic, public, and private-sector experience. He is an expert in accounting regulations, and has extensive experience in audits, risk management, governance and oversight. He was a member of the New Brunswick Legislative Assembly from 1993 to 2003 and held three different cabinet posts, including Minister of Finance from 1999 to 2001. Prior to entering politics, Norman was Assistant Dean of the MBA Programme and an Associate Professor at the University of New Brunswick as well as a partner in the accounting firm Shannon, Betts & Buffett.
Norman received a PhD in Management with a concentration in accounting and finance from Queen’s University School of Business in 1992 and a Bachelor of Business Administration from the University of New Brunswick in 1978. He also holds a Fellow Chartered Accountant designation.
Dave Rosa
Independent Board Member
Dave has spent the past 22 years in a variety of positions in the medical device industry. He was appointed CEO of Sunshine Heart in October of 2009 and served as President and Chief Executive Officer through November 2015. Dave took Sunshine Heart Public on NASDAQ in 2012 and raised over $120M during his tenure. The Company achieved a number of clinical, regulatory and research and development milestones under his leadership. Prior to joining Sunshine Heart, he was President and CEO of Milksmart, Inc., a privately held company developing a unique stent-like agricultural technology that increases milking efficiencies, output and quality. From 2004-2008, Dave was Vice President of Global Marketing for Cardiac Surgery and Cardiology at St. Jude Medical, Inc. While at St. Jude, he developed the strategic pan for the newly formed interventional division, directed the launch of several new products and oversaw the acquisition and integration of Velocimed, a privately held company in the cardiovascular space.
From 1999-2004, Dave held executive management positions at privately held A-Med Systems, Inc., an emerging medical technology company that developed ventricular assist devices (pVADs) for acute heart failure. His roles included Vice President of Marketing and Sales, Chief Operating Officer and Chief Executive Officer. From 1995- 1999, Dave served as Product Manager for Angioplasty Balloons and Director of Intravascular Ultrasound at SCIMED Life Systems, a privately held company that was engaged in the development, manufacture and marketing of medical devices to treat cardiovascular disease. SCIMED was acquired by Boston Scientific Corporation and now operates as a subsidiary of Boston Scientific. Dave holds an M.B.A. in Marketing Management from Duquesne University and a B.S. in Commerce and Engineering from Drexel University.
Patricia Kennedy
Independent Board Member
Patricia has spent over 25 years in a variety of global sales and distribution positions in the medical device industry, focusing on the field of Electrophysiology. In 2015, she founded and is currently the Managing Director of PJM Medical Consultants, supporting medical device companies entering the international market with market entry and product commercialization strategies. From 2008 to 2015, Pat served as VP – International and General Manager for Atricure, Inc. She achieved significant sales, market development and clinical science milestones, while defining strategic plans and pursuing technology development and acquisitions. From 2001 to 2008, Pat worked with Stereotaxis, Inc. in numerous executive positions including Worldwide VP – Clinical Services and VP – International Sales and Marketing.
Prior to Stereotaxis, Pat worked with EP MedSystems as International Marketing Manager for defibrillation and diagnostic catheters. She began her career in the medical industry at Boston Scientific from 1992 to 1997 while serving in several positions, including Sales and International Product Manager for EPT. Pat has earned a Bachelor of Science Degree in Marketing from the University of Florida and a Bachelor of Science in Nursing degree from the University of North Florida.
Steve Salmon
Independent Board Member
Steve Salmon has expertise in business development transactions, market development, finance, technology, and capital market. His most recent experience was as a Venture Capitalist for Latterell Venture Partners, where he managed a number of portfolio companies. Prior to becoming a VC, Mr. Salmon was a founder of Ensure Medical and Integrated Vascular Systems, both of which were successfully exited through acquisition. He also served as the CEO of Revascular which was also acquired. Mr. Salmon brings to Biotricity his deep expertise in raising capital, facilitating growth, and driving long term value.
John Ayanoglou
Chief Financial Officer
John has over 25 years of accounting, finance, and operations experience, having previously served as CFO of several public companies and a regulated financial institution. As CFO of Equitable Group, Mr. Ayanoglou raised over $130 million of regulatory capital in common, preferred, and debenture offerings over successive transactions, all during a period of extreme capital market turbulence, helping to successfully grow assets under administration from $6.1 billion to over $10 billion to facilitate Equitable Bank in becoming Canada’s ninth largest bank. He was also the CFO and Corporate Secretary of Xceed Mortgage Corporation, where he partnered with an entrepreneurial top team to grow the business through the successful implementation of warehouse financing lines and other securitization programs including public term securitizations of over $700 million.
John has extensive experience as a trusted business advisor working with management teams and boards on business acquisition and consolidation, risk mitigation and turnaround, to establish efficient, effective operations. He is a CA, CPA with a Bachelor of Commerce degree in Finance and Economics from the University of Toronto and his ICD.D designation from the Institute of Corporate Directors and the Rotman School of Business.
Amir Ali
Chief Development Officer
Amir is a serial entrepreneur with over 20 years of experience in launching companies and producing innovative products, services, and solutions. Amir is sought after for his expertise in transforming R&D technologies into products based on market needs, commercializing products for volume production, and establishing global distribution channels across the world.
Prior to joining Biotricity, Amir served as CEO at AT Labs Inc., where his team pioneered the concept of wireless mobile thin clients to access secure enterprise applications through the cloud. The efficacy of the robust solutions resulted in customers from healthcare, government, enterprise, and retail sectors, most of which spanned thousands of locations across the United States. His experience in developing such novel technologies has put him at the forefront of companies that have grown in revenue from zero to over $160 million. In his own ventures, he has worked with many leading industrial and financial institutional funders including Jafo, Bay Partners, and JP Morgan with whom he has raised over $35 million.
Vice President, Engineering
Spencer is an expert in wireless communication and has worked with the United States Department of Defense. His academic career was highlighted by various innovative projects and designs. Spencer obtained his undergraduate degree while balancing a full-time job at Flightline Systems Ultra Electronics, a small defense contractor. His specialties are in the design and implementation of radio frequency, digital, analog, embedded software and PC software. Spencer also has experience in working with marketing, sales, and manufacturing teams in order to develop products for large-scale output. Spencer has a Bachelor’s degree in Computer Engineering from Rochester Institute of Technology.
Advisory Board
Thomas C. Nelson
Nelson serves as the president of Share Our Strength, a national nonprofit that is ending childhood hunger in America. Prior to joining Share Our Strength in 2011, Nelson was chief operating officer for AARP, where he led the build-out of its state strategy in all 53 US states and territories. In addition to serving as an adjunct professor at Georgetown University’s McDonough School of Business, Nelson has a long history of service to civic organizations, including his current position on the board of directors of ProInspire. Other board positions include, the board of counselors for the Davis Gerontology School at the University of Southern California; the board of the Certified Financial Planner Board of Standards; and the board of Community Wealth Partners. Nelson holds a Ph.D. from the University of Southern California and an M.A. from Columbia University.
John Rother, JD
Mr. Rother is President and CEO of the National Coalition on Health Care, America’s oldest and most diverse group working to achieve comprehensive health system change. Prior to joining the Coalition in 2011, Rother worked at AARP for over 27 years as the Executive Vice President for Policy, Strategy, and International Affairs where he led the development of AARP’s policy positions and advocacy strategies. Under his leadership, AARP engaged in public policy research and analysis, public education, and advocacy on health and retirement issues at the federal, state and international levels. Rother wrote numerous articles and was a frequent speaker on health, retirement security, the federal budget, and the boomer generation.
Prior to that, Rother was Staff Director and Chief Counsel for the U.S. Senate Special Committee on Aging under the direction of Chairman John Heinz and served as Special Counsel for Labor and Health to U.S. Senator Jacob Javits. He has consistently been named as one of the most powerful people in Healthcare and in 2010 received the Robert Ball Award for Outstanding Achievements in Social Insurance from the National Academy of Social Insurance for “lifetime advocacy to strengthen Social Security and Medicare.”
Daniel Sands, MD, MPH
Dr. Sands is passionate about healthcare transformation, non-visit based care, collaboration in healthcare, and participatory medicine. He spent six years at Cisco, as chief medical informatics officer, where he provided both internal and external health IT leadership and helped key customers with business and clinical transformation using IT. Sands’ prior position was chief medical officer for Zix Corporation, a leader in secure e-mail and e-prescribing. Sands also spent 13 years at Beth Israel Deaconess Medical Center in Boston, where he developed and implemented numerous systems to improve clinical care delivery and patient engagement.
He has earned degrees from Brown University, Ohio State University and the Harvard School of Public Health, and trained at Boston City Hospital and Boston’s Beth Israel Hospital. Sands currently holds an academic appointment at Harvard Medical School and maintains a primary care practice in which he makes extensive use of health IT. He works as a consultant for a variety of innovative companies. Sands is the recipient of numerous health IT awards, has been elected to fellowship in both the American College of Physicians and the American College of Medical Informatics, and is a founder and co-chair of the board of the Society for Participatory Medicine. In 2009, he was recognized by HealthLeaders Magazine as one of “20 People Who Make Healthcare Better.”
Sincerely,

DISCLAIMER
THIS WEBSITE/NEWSLETTER IS A WHOLLY OWNED SUBSIDIARY OF ONE22 MEDIA, LLC, HEREIN REFERRED TO AS O22, LLC
OUR REPORTS/RELEASES ARE A COMMERCIAL ADVERTISEMENT AND ARE FOR GENERAL INFORMATION PURPOSES ONLY. WE ARE ENGAGED IN THE BUSINESS OF MARKETING AND ADVERTISING COMPANIES FOR MONETARY COMPENSATION. WE HAVE BEEN COMPENSATED A FEE OF TEN THOUSAND USD FOR A ONE DAY BTCY AWARENESS CAMPAIGN BY A THIRD PARTY, LEGENDS MEDIA, LLC. WE HAVE PREVIOUSLY BEEN COMPENSATED A FEE OF SIXTY EIGHT THOUSAND USD FOR FOUR PREVIOUS BTCY AWARENESS CAMPAIGNS BY THE SAME THIRD PARTY, LEGENDS MEDIA, LLC. NEVER INVEST IN ANY STOCK FEATURED ON OUR SITE OR EMAILS UNLESS YOU CAN AFFORD TO LOSE YOUR ENTIRE INVESTMENT. THE DISCLAIMER IS TO BE READ AND FULLY UNDERSTOOD BEFORE USING OUR SERVICES, JOINING OUR SITE OR OUR EMAIL/BLOG LIST AS WELL AS ANY SOCIAL NETWORKING PLATFORMS WE MAY USE.PLEASE NOTE WELL: O22 LLC AND ITS EMPLOYEES ARE NOT A REGISTERED INVESTMENT ADVISOR, BROKER DEALER OR A MEMBER OF ANY ASSOCIATION FOR OTHER RESEARCH PROVIDERS IN ANY JURISDICTION WHATSOEVER.RELEASE OF LIABILITY: THROUGH USE OF THIS WEBSITE VIEWING OR USING YOU AGREE TO HOLD O22, LLC, ITS OPERATORS OWNERS AND EMPLOYEES HARMLESS AND TO COMPLETELY RELEASE THEM FROM ANY AND ALL LIABILITY DUE TO ANY AND ALL LOSS (MONETARY OR OTHERWISE), DAMAGE (MONETARY OR OTHERWISE), OR INJURY (MONETARY OR OTHERWISE) THAT YOU MAY INCUR. THE INFORMATION CONTAINED HEREIN IS BASED ON SOURCES WHICH WE BELIEVE TO BE RELIABLE BUT IS NOT GUARANTEED BY US AS BEING ACCURATE AND DOES NOT PURPORT TO BE A COMPLETE STATEMENT OR SUMMARY OF THE AVAILABLE DATA. O22 LLC ENCOURAGES READERS AND INVESTORS TO SUPPLEMENT THE INFORMATION IN THESE REPORTS WITH INDEPENDENT RESEARCH AND OTHER PROFESSIONAL ADVICE. ALL INFORMATION ON FEATURED COMPANIES IS PROVIDED BY THE COMPANIES PROFILED, OR IS AVAILABLE FROM PUBLIC SOURCES AND O22, LLC MAKES NO REPRESENTATIONS, WARRANTIES OR GUARANTEES AS TO THE ACCURACY OR COMPLETENESS OF THE DISCLOSURE BY THE PROFILED COMPANIES. NONE OF THE MATERIALS OR ADVERTISEMENTS HEREIN CONSTITUTE OFFERS OR SOLICITATIONS TO PURCHASE OR SELL SECURITIES OF THE COMPANIES PROFILED HEREIN AND ANY DECISION TO INVEST IN ANY SUCH COMPANY OR OTHER FINANCIAL DECISIONS SHOULD NOT BE MADE BASED UPON THE INFORMATION PROVIDED HEREIN. INSTEAD O22, LLC STRONGLY URGES YOU CONDUCT A COMPLETE AND INDEPENDENT INVESTIGATION OF THE RESPECTIVE COMPANIES AND CONSIDERATION OF ALL PERTINENT RISKS. READERS ARE ADVISED TO REVIEW SEC PERIODIC REPORTS: FORMS 10-Q, 10K, FORM 8-K, INSIDER REPORTS, FORMS 3, 4, 5 SCHEDULE 13D.O22, LLC IS COMPLIANT WITH THE CAN SPAM ACT OF 2003. O22, LLC DOES NOT OFFER SUCH ADVICE OR ANALYSIS, ANDO22, LLC FURTHER URGES YOU TO CONSULT YOUR OWN INDEPENDENT TAX, BUSINESS, FINANCIAL AND INVESTMENT ADVISORS. INVESTING IN MICRO-CAP AND GROWTH SECURITIES IS HIGHLY SPECULATIVE AND CARRIES AND EXTREMELY HIGH DEGREE OF RISK. IT IS POSSIBLE THAT AN INVESTORS INVESTMENT MAY BE LOST OR IMPAIRED DUE TO THE SPECULATIVE NATURE OF THE COMPANIES PROFILED.THE PRIVATE SECURITIES LITIGATION REFORM ACT OF 1995 PROVIDES INVESTORS A SAFE HARBOR IN REGARD TO FORWARD-LOOKING STATEMENTS. ANY STATEMENTS THAT EXPRESS OR INVOLVE DISCUSSIONS WITH RESPECT TO PREDICTIONS, EXPECTATIONS, BELIEFS, PLANS, PROJECTIONS, OBJECTIVES, GOALS, ASSUMPTIONS OR FUTURE EVENTS OR PERFORMANCE ARE NOT STATEMENTS OF HISTORICAL FACT MAY BE FORWARD LOOKING STATEMENTS. FORWARD LOOKING STATEMENTS ARE BASED ON EXPECTATIONS, ESTIMATES, AND PROJECTIONS AT THE TIME THE STATEMENTS ARE MADE THAT INVOLVE A NUMBER OF RISKS AND UNCERTAINTIES WHICH COULD CAUSE ACTUAL RESULTS OR EVENTS TO DIFFER MATERIALLY FROM THOSE PRESENTLY ANTICIPATED. FORWARD LOOKING STATEMENTS IN THIS ACTION MAY BE IDENTIFIED THROUGH USE OF WORDS SUCH AS PROJECTS, FORESEE, EXPECTS, WILL, ANTICIPATES, ESTIMATES, BELIEVES, UNDERSTANDS, OR THAT BY STATEMENTS INDICATING CERTAIN ACTIONS & QUOTE; MAY, COULD, OR MIGHT OCCUR. UNDERSTAND THERE IS NO GUARANTEE PAST PERFORMANCE WILL BE INDICATIVE OF FUTURE RESULTS.IN PREPARING THIS PUBLICATION,O22, LLC HAS RELIED UPON INFORMATION SUPPLIED BY ITS CUSTOMERS, PUBLICLY AVAILABLE INFORMATION AND PRESS RELEASES WHICH IT BELIEVES TO BE RELIABLE; HOWEVER, SUCH RELIABILITY CANNOT BE GUARANTEED. INVESTORS SHOULD NOT RELY ON THE INFORMATION CONTAINED IN THIS WEBSITE. RATHER, INVESTORS SHOULD USE THE INFORMATION CONTAINED IN THIS WEBSITE AS A STARTING POINT FOR DOING ADDITIONAL INDEPENDENT RESEARCH ON THE FEATURED COMPANIES. THE ADVERTISEMENTS IN THIS WEBSITE ARE BELIEVED TO BE RELIABLE, HOWEVER, O22, LLC AND ITS OWNERS, AFFILIATES, SUBSIDIARIES, OFFICERS, DIRECTORS, REPRESENTATIVES AND AGENTS DISCLAIM ANY LIABILITY AS TO THE COMPLETENESS OR ACCURACY OF THE INFORMATION CONTAINED IN ANY ADVERTISEMENT AND FOR ANY OMISSIONS OF MATERIALS FACTS FROM SUCH ADVERTISEMENT. O22, LLC IS NOT RESPONSIBLE FOR ANY CLAIMS MADE BY THE COMPANIES ADVERTISED HEREIN, NOR IS O22, LLC RESPONSIBLE FOR ANY OTHER PROMOTIONAL FIRM, ITS PROGRAM OR ITS STRUCTURE. 022, LLC IS NOT AFFILIATED WITH ANY EXCHANGE, ELECTRONIC QUOTATION SYSTEM, THE SECURITIES EXCHANGE COMMISSION OR FINRA.