_______
OUR NEW PROFILE IS: (Nasdaq: JUPW)
JUPW recently executed a share buyback program through which we retired approximately 11% of the shares outstanding
In the second quarter of 2022 revenues surpassed $3 million which is a five-fold increase over the same period in 2021 The Company generated $1,569,925 in revenues for the 3rd quarter compared to $687,928 for the same period in 2021.
Jupiter Wellness’s wholly-owned subsidiary, SRM Entertainment, received $3.6 million in guaranteed purchase orders from the world’s largest amusement parks.
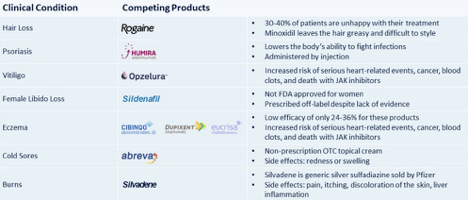
JUPW has licensing deals with Taisho, a $2.6 billion revenue company and Japan’s leading seller of minoxidil products. A $300k up-front payment was received with 3% royalties on net sales. They expect to launch the product commercially in 2023. Also Licensed to India-based Cosmofix Technology and Sanpelle.
CHECK OUT THE INVESTOR PRESENTATION HERE
__________________
Hello Everyone,
October and November have certainly been eventful months.
We have been able to release some incredible profiles that went on to make double and even triple digit gains.
Our last profile moved over 20% from the open on the session with way above average interest.
Today’s profile is one that we recently looked at earlier this month before it went on a 38% run over the next 5 sessions.
Breast cancer is one of the top talked about cancers and has now surpassed lung cancer as the most commonly diagnosed cancer globally as of 2021, according to the American Cancer Society and the International Agency for Research on Cancer.
Several pharmaceutical companies including Bristol-Myers Squibb Co. (NYSE: BMY), Merck & Co. Inc. (NYSE: MRK), Medivation Inc. (NASDAQ: MDVN), and Pfizer Inc. (NYSE: PFE) have reported developing breakthrough drugs to treat breast cancer over the years. Yet the adverse effects of cancer treatment continue to cause concern and unhappiness for breast cancer survivors.
Following breast cancer treatment or surgery, some women experience a number of side effects, including a change in body weight, tightness of the skin, changes in sensation, phantom breast sensations (in surgery patients), and loss of sexual desire (libido).
These side effects are little talked about, but they matter to many women across the world and one Florida-based biopharma company is paying attention to this big market.
Trading just above $1, Jupiter Wellness, Inc. (NASDAQ: JUPW) has licensed a breakthrough product with a novel mechanism of action for women’s sexual wellness.
The drug, called JW-500, could be a game-changer in women’s wellness and could put JUPW on the map as they plan to file for a pre-IND meeting with the US FDA within the next year. They also want to seek Orphan Drug Designation.
Considering the massive size of the market for breast cancer survivors, this is just one reason to put Jupiter Wellness, Inc. (NASDAQ: JUPW) high on your radar!
Jupiter Wellness, Inc. (NASDAQ: JUPW) is a diversified company supporting health and wellness through the research and development of over-the-counter (OTC) products and intellectual property.
The company supports health and wellness through the research and development of over the counter (OTC) products and intellectual property. The company’s product pipeline addresses a range of conditions, including hair loss, eczema, burns, and sexual wellness.
Revenue is generated through the sales of OTC and consumer products, contract research agreements, and licensing royalties.
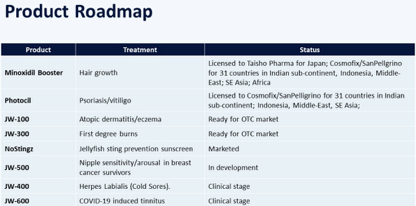
JUPW is targeting tremendous markets with well-known rivals that include Johnson & Johnson owned Rogaine, Abbott Laboratories ownedHumira, and Pfizer’s Sildenafil.
In clinical trials, the company’s eczema cream was shown to clear or reduce symptoms after just 2 weeks of daily use.
Initial results were so impressive they suggest that JW-100 may potentially prove superior to existing prescription drugs as an OTC treatment with virtually no side effects!
Thus, a Phase 3, double-blind placebo-controlled, multi-center trial is underway to directly evaluate the superiority of JW-100 to Eucrisa (an FDA-approved topical drug) for the treatment of mild-to-moderate eczema. Positive results in that clinical trial could lead to an IND filing with the FDA.
Indications and Competitors
Hair Loss (Minoxidil Booster) is a Topical treatment designed to improve Minoxidil efficacy.
JUPW reports minoxidil is the only FDA-approved topical drug for treating common hair loss (androgenetic alopecia)!
*****A RECENT SHAREHOLDER UPDATE FROM THE CEO*****
Dear Fellow Shareholders,
I am motivated to write to you today to outline the launch of Jupiter Wellness 2.0 underway. We started as a CBD/sun care company developing products with the potential to protect users from the sun while making them healthier. Those products were founded on science and the belief we could create research-backed solutions to enhance the well-being of our customers. Today we are refocusing our scientific approach on developing products to address a range of conditions, including hair loss, eczema, burns, and sexual wellness. We spent the last year developing, filing for patents, and clinically testing our core products:
- JW-100 – Topical treatment of eczema (in development)
- RJ-101 – Topical treatment for female sexual wellness (in development)
- Minoxidil Booster – Topical treatment designed to improve Minoxidil efficacy (on the market)
- Photocil – Topical treatment for psoriasis and vitiligo (on the market)
- JW-300 – Topical treatment of first-degree burns and sun exposure (in development)
- NoStingz – Topical protection from jellyfish, sea lice, and UVA/UVB rays (on the market)
- JW-400 – Topical treatment of cold sores (in development)
In August we reported our strongest quarter of earnings to date. In the second quarter of 2022, we posted revenues that surpassed $3 million, a five-fold increase over the same period in 2021. We also substantially reduced our general and administrative expenses over the prior year. Through our continued efforts to drive value to our shareholders in the face of an unforgiving equity market, we executed a share buyback program through which we retired approximately 11% of the shares outstanding, 2,690,354 shares, at an average price of $1.0321 per share.
In clinical trials, our eczema cream was shown to clear or reduce symptoms after just 2 weeks of daily use. Our initial results were so impressive they suggest that JW-100 may potentially prove superior to existing prescription drugs as an OTC treatment with virtually no side effects. Thus, a Phase 3, double-blind placebo-controlled, multi-center trial is underway to directly evaluate the superiority of JW-100 to Eucrisa (an FDA-approved topical drug) for the treatment of mild-to-moderate eczema. Positive results in that clinical trial could lead to an IND filing with the FDA. We have already had a positive Pre-IND meeting with the FDA to determine the development pathway to eventual FDA approval. Alternatively, we have plans to bring the product to market as a skin care system that would not require FDA approval of the CBD component.
RJ-101 was born out of clinical trials designed to establish a topical treatment for the restoration of nipple sensitivity for breast augmentation Patients, in addition to patients who had undergone chemotherapy or lumpectomy surgery following a cancer diagnosis. During those early studies, women reported not only increased sensitivity, but also better sex and increased libido. We plan to initially develop this product for the treatment of nipple neuropathies in breast cancer patients through the filing of an IND.
In addition to expanding our product lines, we continue to grow our contract research (CRO) business through the acquisition of Applied Biology and Ascent Clinical Research assets. Included with these assets are several ongoing contracts with milestone payments for our company fast approaching. In 2021, Applied Biology recognized revenues of approximately $8m with $4m in EBITDA. Ascent Clinical Research assets included ongoing contracts worth $3m in annual revenues which we purchased for 5% royalties on future net revenues generated by the assets.
In the first half of this year, we also built a foundation on which to generate significant revenues through the licensing of our intellectual property (IP). We signed agreements to license our minoxidil booster to Taisho, a $2.6 billion revenue company and Japan’s leading seller of minoxidil products. A $300k up-front payment was initially received to be followed by 3% royalties on future net sales. Taisho plans on launching the product commercially in 2023.
In India, we inked a deal with Cosmofix Technovation Pvt Ltd and Sanpellegrino Cosmetics to license our minoxidil booster and our Photocil products. We received a $20k up-front payment with 10% royalties on future net sales which are also expected to commence in 2023. Additional licensing opportunities for these products are being pursued primarily in overseas markets to avoid cannibalizing our own domestic sales.
To execute our plans and goals we have begun expanding our corporate team in Jupiter, FL. We recently added a senior project manager to oversee our manufacturing processes and improve the efficiency of the entire team. Our marketing and communications teams have also seen recent additions. We hired a new head of marketing and have begun to develop market strategies and go-to-market plans for each upcoming product launch. Lastly, we have added members to our communications team to expand our investor outreach and improve the flow and frequency of communications to the investment world.
As we continue to advance our product development pipeline, I believe we are extremely well positioned to advance Jupiter Wellness forward in our markets.
With numerous milestones ahead of us, I am excited to see Jupiter Wellness achieve its potential. I believe in our mission to enhance the well-being of our customers and increase value for our shareholders.
We thank you for your continued support of Jupiter Wellness.
Sincerely,
/s/ Brian John
Chief Executive Officer, Jupiter Wellness
Zooming in on the Women’s Sexual Wellness Category
Female audiences have been routinely placed in the shadows by major CPG companies when it comes to addressing and serving their core sexual wellness needs.
JUPW is taking notice of this key and very important demographic that represents massive spending power.
One of the most exciting developments the company has is JW-500 – which will address a wide range of clinical indications including: female orgasmic disorder, nipple neuropathies after breast augmentation, and orgasmic adverse events in breast cancer survivors.

The company has reported that its proposed drug, JW-500, when topically applied to the nipple-areola area of the breast, contracts the smooth muscle, thus erecting the nipple and increasing its sensitivity.
JUPW also reports that clinical trials of JW-500 have shown efficacy and safety in improving female nipple sensitivity. Further testing is being conducted to quantify and qualify enhanced sexual arousal, vaginal lubrication, and orgasm response in healthy women and women suffering from nipple naturopathy following breast cancer treatment.
“This treatment has the potential to address a wide range of clinical indications, including nipple neuropathies following breast augmentation in breast cancer survivors. We are excited to complete the research and development of this product and to bring this novel approach to the market. I believe this product may not only be an excellent treatment for people with breast cancer but potentially as a women’s sexual health product as well.”
- Jupiter Wellness CEO Brian John
As mentioned before, JUPW has licensed this breakthrough product. A recent press release, has said, “The exclusive license includes issued patents and technology, including formulations.”
Trial for Potential New Treatment for Tinnitus is Initiated
JUPW announced in October the initiation of a triple-blinded clinical study evaluating a possible treatment for tinnitus.
This could be monumental, as to date, there is no effective treatment exists for tinnitus!
The pandemic has brought on many cases of tinnitus and has established a wide market for the company to also zoom in on.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative viral pathogen leading to the COVID-19 pandemic. In 2022, after three successive waves of the pandemic, much of the human population has been exposed to the SARS-CoV-2 virus. During the two years since the pandemic ensued, multiple reports emerged of patients experiencing COVID-19 emergent symptoms lasting longer than 4 weeks since onset. Up to 15% of patients recovering from COVID-19 experience post-acute COVID-19 induced tinnitus.
During the pandemic, JUPW scientists discovered a novel pathway for the treatment of COVID-19 induced tinnitus and possibly subjective tinnitus. The discovery led to a patent recently filed by the company for the use of dopamine receptor modulation for the treatment of tinnitus.
The Company has also launched a study to investigate if dopamine receptor modulation can be used effectively to treat COVID-19 induced tinnitus (https://clinicaltrials.gov/ct2/show/NCT05507372).
In the second quarter of 2022, the company posted revenues that surpassed $3 million, a five-fold increase over the same period in 2021. They also substantially reduced our general and administrative expenses over the prior year.
“We have had a great start to the year. We’re coming off a record quarter for revenue and hitting several key milestones for our company. We are executing a pivot back to our roots and putting a renewed focus on the science that founded this company. We spent the last year developing, patent protecting, and clinically testing our core products. We expanded our CRO business with the acquisitions of Applied Biology. We signed several agreements to license our products, including Taisho a $2.6 billion revenue company and Japan’s leading seller of minoxidil products. Lastly, we are turning our focus now to the development and launch of our eczema and sexual wellness products.”
- Brian John, CEO of Jupiter Wellness
In the first half of this year, the company also built a foundation on which to generate significant revenues through the licensing of their intellectual property (IP).
JUPW signed agreements to license our minoxidil booster to Taisho, a $2.6 billion revenue company and Japan’s leading seller of minoxidil products. A $300k up-front payment was initially received to be followed by 3% royalties on future net sales. Taisho plans on launching the product commercially in 2023!
In recent years the world has experienced an explosion of wellness startups.
A recent research report by Global Wellness Institute noted, “As we emerge from the pandemic, GWI predicts that the wellness economy will return to its robust growth. We project 9.9% average annual growth, with the wellness economy reaching nearly $7.0 trillion in 2025.”
JUPW’s clinical pipeline is competing against some of the biggest names in skin care, hair loss, and sexual wellness.
As an emerging company on the NASDAQ and at around $1, their story seems to be only now unfolding…
Jupiter Wellness, Inc. (NASDAQ: JUPW) is proving to be an exciting player to watch in this almost $7 trillion-dollar wellness industry with many exciting acquisitions and products geared at massive markets!









