
OUR NEW PROFILE IS: (NASDAQ: CMND)
__________
Clearmind Medicine is pleased to announce that its ordinary shares have been uplisted to @Nasdaq under the symbol “CMND”. Trading of the Company’s ordinary shares on Nasdaq commenced on Nov 15th, 2022.#nasdaq #shares #uplisted #MEAI #biotechnology #psychedelic $CMND pic.twitter.com/uxtuAtecBZ
— Clearmind Medicine (@ClearmindCMND) November 18, 2022
CMND trades in Canada (CSE: CMND) as well as Germany (FWB: CWY)
READ THE INVESTOR PRESENTATION HERE
_______________________
Hello Everyone,
We have another exciting profile for you for for today’s session. This one operates in a unique, niche sector with a bright future ahead of it. With a recent uplisting to the NASDAQ, Clearmind Medicine, Inc. (NASDAQ: CMND) is a company to look at closely! The world is in a mental health crisis, with near all-time level highs of depression, PTSD, anxiety, and addiction. The pandemic has negatively affected many people, impacting their mental health on many levels. The current mental health treatments available, such as SSRIS for depression and rehab for addiction, may be effective, but also leave far too many people behind. Plus, the treatment for these conditions is massive, raking in billions of dollars a year, even if they aren’t effective. There needs to be a solution and the growing psychedelics movement might be it. Early scientific evidence is showing that treatments such as MDMA therapy for PTSD, psilocybin therapy for depression, and ketamine therapy for addictions, are much more effective than current treatments!
Are we looking at another sector before it goes mainstream. We all know how many millionaires were made from trading MJ companies made trading these things early on. It is certainly worth noting that voters passed the 2020 Oregon Ballot Measure 109, making it the first state to both decriminalize psilocybin and also legalize it for therapeutic use. Colorado followed with the 2022 Colorado Ballot Measure 122.
A report from Data Bridge Market Research estimates that the global psychedelic drugs market will reach $6.4 billion by 2029. Such an expansion would mean a compounded annual growth rate (CAGR) of well over 13% between 2022 and 2029. Clearmind Medicine, Inc. (NASDAQ: CMND) is a psychedelic pharmaceutical biotech company focused on the discovery and development of novel psychedelic-derived therapeutics to solve widespread and underserved health problems, including alcohol use disorder. Its primary objective is to research and develop psychedelic-based compounds and attempt to commercialize them as regulated medicines, foods or supplements.
CATALYSTS
Clearmind’s flagship treatments are focused on Alcohol Use Disorder (AUD), which is incredibly common. It varies from mild to excessive, and describes a person’s inability to restrict their alcohol consumption, despite negative social, occupational, or health consequences.
- Alcohol consumption is the 3rd leading preventable cause of death in the US.
- The yearly cost of excessive alcohol use in the US reached $249Bn in 2010.
- Alcohol consumption contributes to 3 million deaths each year globally… In addition to disabilities and poor health of millions of people. – World Health Organization
—–
The Solution: MEAI (5-Methoxy-2-aminoindane)
5-Methoxy-2-aminoindane (MEAI) is a psychoactive molecule, exerting a euphoric alcohol-like experience and a reduced desire to consume alcoholic beverages. It shows a good safety profile in pre-clinical studies, with potential to change the lives of millions who struggle to drink in moderation.
Breaking the Cycle
We believe that MEAI breaks the vicious binge-drinking cycle at the decision point to drink more alcohol, by potentially innervating neural pathways such as 5-HT1A that lead to “sensible behaviour.”
Non-Addictive
Anecdotal reports and pre-clinical in-vivo results indicate the self-limiting property of MEAI—unlike traditional treatments.
Expansive Potential
The literature shows that 5-HT1A receptors are associated with controlling craving behavior across the board. This indicates that MEAI may have a wide range of applications beyond binge drinking.
—–
Successful Pre-clinical Results In MEAI Treatment For Alcohol Consumption
Pre-clinical trial demonstrated a high safety profile in addition to a significant suppressive effect on alcohol consumption.
- Clearmind conducted preclinical trials, training groups of mice to consume alcohol for five weeks
- The groups were then given a daily dose of MEAI, with intermittent access to alcohol and water
- After two weeks, researchers found a significant reduction in alcohol consumption in mice receiving MEAI
- The untreated control group showed no significant reduction in alcohol consumption
- No SAEs or treatment-related histological changes were observed amongst all MEAI treatment groups compared to control naive and vehicle animals in all the examined organs
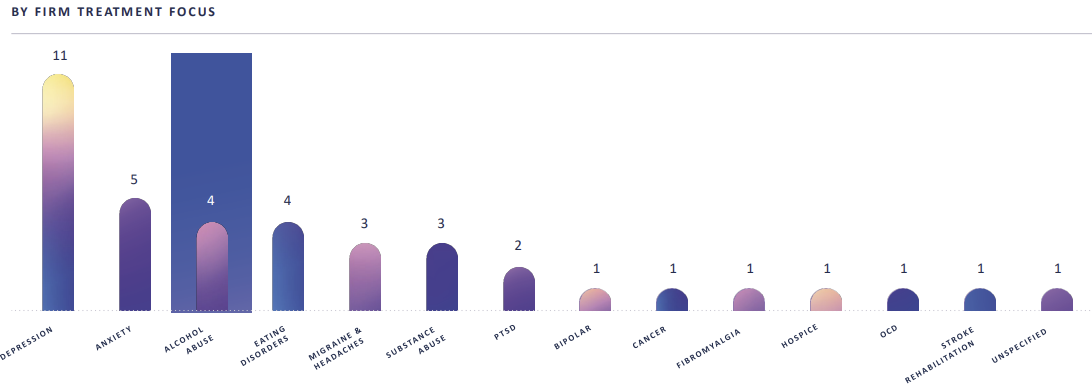
Low Competitive Intensity
Only 3 other psych-e-delic companies are working on alcohol abuse problems:
The Company’s Competitive Advantage Most companies in the psych-e-delics field target depression and anxiety. Clearmind focuses on binge behaviors and addictions: A huge untapped market Most companies develop classic psych-e-delics for which intellectual property is hard to file and approve. Clearmind is the sole developer of MEAI and has a robust portfolio of protected intellectual property Most psych-e-delic treatments are designed to be adjuncts to psychotherapy. MEAI is designed to be self administered, due to its high safety potential, and not in conjunction to psychotherapy —– Expected Timeline: |
|
Clearmind Announces Initiation of CMND-100 Manufacturing Program to Address its Upcoming Clinical Trialhttps://t.co/64KeL8Yg3I#biotechnology #psychedelic #MEAI #alcoholusedisorder #clearmind #brainhealth #stocks #trading #nasdaq #uplisted pic.twitter.com/VfkPYhPbwS
— Clearmind Medicine (@ClearmindCMND) November 25, 2022
Clearmind Announces Initiation of CMND-100 Manufacturing Program to Address its Upcoming Clinical Trial
VANCOUVER, November 23, 2022 — Clearmind Medicine Inc. (Nasdaq: CMND), (CSE: CMND), (FSE: CWY) (“Clearmind” or the “Company“), a biotech company focused on discovery and development of novel psychedelic-derived therapeutics to solve major undertreated health problems, today announced the initiation of clinical batches of production of its novel psychedelic-derived drug candidate, the MEAI-based molecule- CMND-100.

The produced batches will be used in the Company’s upcoming first in human (FIH) clinical trial evaluating the proprietary drug candidate compound CMND-100 for the treatment of Alcohol Use Disorder (AUD).
Following MEAI’s synthesis development process, the compound is being produced under GMP (Good Manufacturing Process) conditions to comply with FDA requirements. The clinical batches production is made possible due to prior successful production of MEAI drug substance that was used in the Company’s pre-clinical studies designed to evaluate the safety of its innovative compound.
“Clearmind continues its progress toward FIH clinical trial” said Dr. AdiZuloff-Shani, Clearmind’s Chief Executive Officer. “This milestone joins other achievements we’ve made in a relatively short period. Non-clinical data generated to date, demonstrate that our MEAI- based treatment has the potential to treat broad range of addictions and binge behaviors such as AUD.”
“Like other addictions, AUD is a chronic relapsing brain disorder characterized by an impaired ability to stop or control alcohol use,” she added. “Alcohol abuse is the third most-common preventable cause of death in the United States, where almost 6% struggle with this condition. ”
The Company previously announce that it completed a highly constructive Pre-Investigational New Drug Application (“pre-IND”) meeting with the U.S. Food and Drug Administration (“FDA”) to discuss the development of CMND-100.
Clearmind Applies for Patent to Treat Obesity and Metabolic Syndrome
Patent Application, Based on Research at the Hebrew University, is Latest Result of Ongoing Collaboration with SciSparc Ltd.
VANCOUVER, Sept. 22,2022 — Clearmind Medicine Inc. (CSE: CMND), (OTC Pink: CMNDF), (FSE: CWY0) (“Clearmind” or the “Company“), a biotech company focused on discovery and development of novel psychedelic-derived therapeutics to solve major undertreated health problems, today announced it has filed a provisional patent application related to metabolic syndromes including obesity.
The patent application is another result of the company’s ongoing collaboration with SciSparc Ltd.(NASDAQ: SPRC) (“SciSparc”), a specialty clinical-stage pharmaceutical company focusing on the development of therapies to treat disorders of the central nervous system, and with the Hebrew University of Jerusalem.
The patent application is the third application resulting from the collaboration with SciSparc, referring to the proprietary combination of Clearmind’s MEAI, a novel proprietary psychedelic treatment for various addictions, and SciSparc’s Palmitoylethanolamide (PEA), the active ingredient of its proprietary CannAmide™, which is used for treating obesity and its related metabolic disorders.
“Food addiction and obesity are an epidemic raging in the United States and around the world, yet there are few safe and effective anti-obesity treatments on the market,”said Dr. Adi Zuloff-Shani, Clearmind’s Chief Executive Officer.
“In pre-clinical studies MEAI has shown great potential in its ability to treat different addictions. Certain metabolic syndromes can be associated to addictive behaviors, and we believe combining MEAI with SciSparc’s CannAmide™ may create a valuable tool for treating these conditions.”
The study was conducted as part of Clearmind’s research and development projects with the Hebrew University’s Obesity and Metabolism Laboratory, which is led by Joseph Tam,D.M.D., Ph.D., Associate Professor of Pharmacology at the University’s Institute for Drug Research.
“This new patent application continues Clearmind’s strategy since inception to enhance its IP portfolio to create state-of-the-art psychedelic drug candidates, to better serve patients in need,” said Dr. Zuloff-Shani.
Clearmind Medicine Announces Positive Pre-Clinical Results for Cocaine Addiction Treatment
The dedicated treatment demonstrated a significant decrease in preference for an area associated with cocaine
VANCOUVER, June 7, 2022–Clearmind Medicine Inc. (CSE: CMND), (OTC Pink: CMNDF), (FSE: CWY0) (“Clearmind” or the “company“), a biotech company focused on discovery and development of novel psychedelic-derived therapeutics to solve major undertreated health problems, today announced positive pre-clinical results for its treatment for cocaine addiction using MEAI, its novel psychedelic molecule.
“This exciting research on MEAI could go a long way in helping those who need it,” said Dr. Adi Zuloff-Shani, Clearmind’s ChiefExecutive Officer. “While effective treatments for opioid addictions have been available, this could be the first dedicated treatment for cocaine addiction, which is a global crisis.”
The pre-clinical trial, led by Professor Gal Yadid and his team, from the Gonda Multidisciplinary Brain Research Center located at Bar Ilan University (RamatGan, Israel), was designed to evaluate possible reward-like effects of MEAI, on male Sprague-Dawley rats based on the Conditioned Place Preference (CPP*)model.
Rats previously conditioned with cocaine received either cocaine (at 15mg/kg) or MEAI at doses of 2.5, 5, 10 and 20 mg/kg. Rats treated with MEAI spent less time in the compartment associated with cocaine. Results suggest a potential role for MEAI in abolishing cocaine-induced conditioned place preference and eliminating heightened craving, and that the compound was not itself addictive.The 5 mg/kg dose was found the most effective dose and selected for further study.
The results followed Clearmind’s recent news regarding filing a provisional patent application related to cocaine addiction.
“Once again, MEAI has shown the potential to become a game changer in the field of addiction treatments” Zuloff- Shani added.”We intend to further explore the potential of MEAI as an anti-cocaine addiction treatment.”
*A testing method by which the animals learned to associate between reward and an environmental cue
MANAGEMENT
NEWS
23
November
Clearmind Announces Initiation of CMND-100 Manufacturing Program to Address its Upcoming Clinical Trial
17
November
Clearmind Medicine Announces Closing of US$7.5 Million Public Offering and Uplisting to the Nasdaq Capital Market
24
August
Clearmind Medicine Announces Additional Positive Pre-Clinical Results for its Cocaine Addiction Treatment
7
June
Clearmind Medicine Announces Positive Pre-Clinical Results for Cocaine Addiction Treatment
2
June
Clearmind Medicine and SciSparc Collaboration Yields Another Patent Application for Cocaine Addiction Treatment
Sincerely,
The Viral Stocks Team
DISCLAIMER










