OUR NEW PROFILE IS: (Nasdaq: BSGM)
BioSig Sees Positive Momentum from Sales Pipeline Growth
Completion of more than 2500 procedures
Substantial and growing IP patent portfolio
*****NEW CATALYST***** INSIDER BUYING
—–In the last week or so we have seen the CEO come in and buy roughly 60K shares. Now we are seeing more insider buying from the COO
__________________
DOWNLOAD THE FACT SHEET HERE
GET THE INVESTOR PRESENTATION HERE
__________________________________
Hello Everyone,
We have a new Nasdaq profile that we want to bring to your attention for today.
It has been a while since we took a look at this next one.
Pull up BSGM immediately for today’s session.
BioSig Places Second PURE EP™ System For Evaluation at Cleveland Clinic, a Leading Medical Center of Excellence. Read the full news release here: https://t.co/eDTvqgAcm4 $BSGM pic.twitter.com/OVs5vrqjXk
— BioSig Technologies (@BioSig_Tech) August 24, 2022
BSGM is a medical technology company commercializing a proprietary biomedical signal processing platform designed to improve the electrophysiology (EP) marketplace. With the global EP market projected to reach $10.1B in 2024 with a 11.3% growth rate, BioSig has commenced a targeted commercial release of its PURE EPTM System, generating its first commercial sale in December 2020. The Company’s first product is a computerized system designed to reveal the full range of cardiac signals and to provide physicians with signal clarity during procedures performed to address cardiac arrhythmias. The PURE EPTM System received FDA 510(k) clearance in August 2018. Physicians using BioSig’s PURE EPTM System have successfully completed over 1,700 patient cases to date. Systems are currently installed at multiple locations, including Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Mayo Clinic’s Florida, Minnesota and Arizona campuses, Massachusetts General Hospital, the University of Pennsylvania, Deborah Heart and Lung Center, and Houston Methodist.
BioSig Sees Positive Momentum from Sales Pipeline Growth. Read the full news release here: https://t.co/eVyXdrtYpi $BSGM #MedTech #MedDevice #MedicalTechnology pic.twitter.com/QODI586xra
— BioSig Technologies (@BioSig_Tech) August 16, 2022
BioSig Sees Positive Momentum from Sales Pipeline Growth
Westport, CT, Aug. 16, 2022 (GLOBE NEWSWIRE) —
- Company sees increase in medical centers entering into 60-day evaluation agreements
- Existing customers seeing positive results from PURE EP™ System expected to increase number of units purchased
BioSig Technologies, Inc. (NASDAQ: BSGM) (“BioSig” or the “Company”), a medical technology company advancing electrophysiology workflow by delivering greater intracardiac signal fidelity through its proprietary signal processing platform, today announced that it is seeing positive momentum from the growth of its sales pipeline, and expects to see an increase in enterprise adoption of its PURE EP™ System in the coming months.
Since BioSig’s national commercial launch of its PURE EP™ System on July 1st, 2022, the Company’s commercial pipeline has experienced a steady increase in advanced leads and technology adoption across several key regions and centers of excellence. Under the terms of its new leasing program, the Company recently signed a purchase agreement with Kansas City Heart Rhythm Institute at Overland Park Regional Medical Center. In addition, the Company inked its first master services agreement with one of the largest U.S. healthcare systems.
Among several key regions, BioSig’s PURE EP™ System continues to gain interest in hospitals across the Midwest, including new evaluation agreements with the Cleveland Clinic, a leading Medical Center of Excellence, and an additional installation at a leading medical center in Springfield, IL.
“The demand for minimally invasive catheter-based ablation procedures continues to grow. We believe that market demand is high, and expect to see an acceleration of commercial activity in our quarterly results going forward,” commented Kenneth L. Londoner, Chairman and CEO of BioSig Technologies, Inc.
BioSig’s commercial momentum is supported by its recent decision to streamline the PURE EP™ System evaluation period from 180-360 days to 60-days. The Company has also implemented a new leasing program to help expedite the acquisition of Pure EP’s superior signal processing capabilities and shortens the sales cycle. Consistent with its stated commercial strategy, BioSig is prioritizing the growth of its robust sales team, including the recent appointment of a new sales leader who will cover the COLT states (Colorado, Oklahoma, Louisiana, and Texas).
“By shortening our evaluation period and providing flexible paths to purchase, we are meeting the demands of physicians and supply chain management, ensuring that superior signal processing technology is within reach. We’re pleased to be exploring opportunities for repeat business and additional unit placement with many of our existing accounts,” commented Gray Fleming, Chief Commercialization Officer, BioSig Technologies, Inc.
Looking further ahead, the Company will be participating in several key industry conferences and events, including the 2022 Kansas City Heart Rhythm Symposium, taking place at the end of the month and the Cleveland Clinic Global EP Summit 2022 in September, where BioSig will serve as sponsor at the annual global summit.
The Company is also expanding its clinical research pipeline, including the recent commencement of a physician-initiated research protocol that will analyze the signals acquired by its PURE EP™ System during Radiofrequency (RF) ablation. Led by Dhanunjaya DJ Lakkireddy, MD, Medical Director for the Kansas City Heart Rhythm Institute, the single center study underway at Overland Park Regional Medical Center, is officially registered with clinicaltrials.gov [NCT05464537], and includes 30 participants with paroxysmal atrial fibrillation (AF) undergoing pulmonary vein isolation (PVI).
________
Achievements
- Announced Evaluation Agreement with Cleveland Clinic
- Surpassed 2,500 procedures
- Announced 49 allowed/ issued design and utility patents
- Appointed John Sieckhaus as Chief Operating Officer
- Appointed Gray Fleming as Chief Commercial Officer
- Clinical data acquired by PURE EPTM published in the Journal of Cardiovascular Electrophysiology
- Selected Plexus Corp. as its manufacturing partner
- Announced medical device industry leader to its board of directors
- Unblinding of clinical data from PURE EPTM 2.0 Study showed signal superiority to standard of care
- Achieved first commercial sales at TCAI and various Mayo Clinic sites
Investment Highlights
• World class commercial team
• Achieved first revenues
• Conducted First Clinical Trial with PURE EPTM System
• Significant Insider Ownership
• 10-year Strategic Collaboration with Mayo Clinic
• IP Strategy Led by Sherpa Technology Group and Sterne Kessler Goldstein & Fox. — 49 allowed/issued design and utility patents
• FDA clearance achieved
• Proven Management Team and Board of Directors
• Global and Growing Addressable Market
• Operates Within Rapidly Emerging Field of Bioelectronic Medicine
• High-Growth Sector Earns Innovation Premium, Aggressive M&A
BSGM has made tremendous strides in the placement of their flagship product, PURE EP™ System in clinics across the country this year and has begun to generate revenues, $325K over Q1 and Q2 and I anticipate Q3 results soon.
In the past two weeks, the share price has crossed the 20 day SMA of $2.90 and the 50 SMA of $2.84 while fast closing in on the 200 SMA of $3.63. While this movement indicates the shares are trending, it may also indicate a near term breakout. Remember the 52-week high is $6.14/share. This is the momentum in company valuation we mentioned above.
The company’s flagship PURE EP™ System is an advanced monitoring system currently used primarily for cardiac ablation procedures in patients with AFIB (atrial fibrillation) a quivering or irregular heartbeat (arrhythmia) that can lead to blood clots, stroke, heart failure and other heart-related complications. The PURE EP™ System is an FDA 510(k) cleared non-invasive class II device. BSGM just installed a PURE EP™ System for an evaluation at the HCA Healthcare-operated Medical City Heart Hospital in Dallas, TX. This is the Company’s 17th installation. To date, over 71 physicians have completed over 1600 patient cases with the PURE EP™ System.
In 2020 the number of patient cases was only 425.
Clinical data acquired by the PURE EP™ System in a multi-center study at Texas Cardiac Arrhythmia Institute at St. David’s Medical Center, Mayo Clinic Jacksonville and Massachusetts General Hospital was recently published in the Journal of Cardiovascular Electrophysiology and is available electronically with open access via the Wiley Online Library. I want to note that BSGM has a long-term relationship with the Mayo Clinic in the development of the PURE EP™ System.
Last month BSGM signed a manufacturing and professional services agreement with Plexus Corp. (PLXS). Under the terms of the newly reached agreement, Plexus will bring to market the PURE EP™ System, the Company’s signal processing technology for arrhythmia care, and develop a new product pipeline for BioSig’s subsidiary NeuroClear Technologies, Inc. The signing of this manufacturing agreementis yet another potential catalyst in the near-term growth coming for BSGM.
BSGM maintains a small share structure with 34.9M shares outstanding and 30.4M shares in the public float. The company’s last reported balance sheet at the end of June 2021, indicated zero debt and cash of $15.5M.
NeuroClear
Novel ENG Platform Technology
Our technology aims to address technological deficiencies present in the current electroneurogram recording systems through high-speed recording of biomedical signals, the ability to preserve valuable clinical information and optimization of therapy delivery through closed feedback loop.
Our first product focuses on improving safety and efficacy of renal denervation procedures.
Introducing N-SENSE
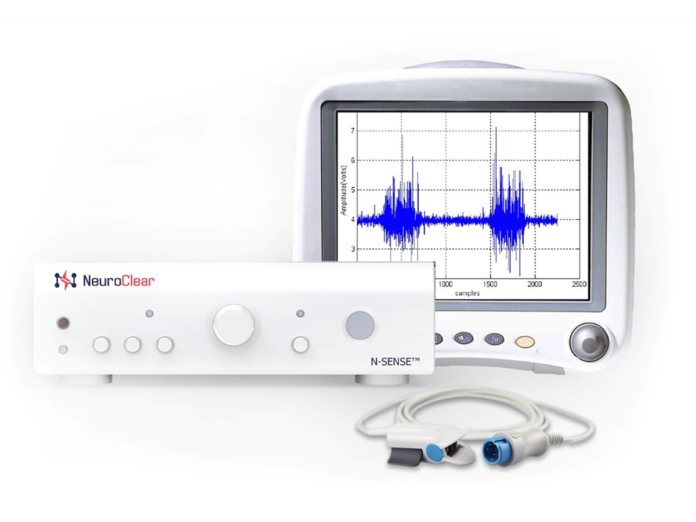
- Multi-channel hardware designed to sense & stimulate nerves.
- Simultaneous stimulation and measure for optimal closed loop feedback system.
- Aid in targeting nerve location.
- Algorithms tailored to specific applications.
- Assess nerve for hyperactivity.
- Catheter-agnostic interface.
Partnerships
We have partnerships with some of the most distinguished organizations and experts in the fields of electrophysiology, intellectual property (IP) strategy, and technology development.
In 2017 we signed a ground-breaking 10-year strategic collaboration with experts at Mayo Clinic to both develop advanced clinical features of the PURE EP™ System and explore new disease areas and applications. We expect our collaboration to result in joint patent filings and licensing opportunities. Our research program with Mayo Clinic is run under the leadership of Samuel J. Asirvatham, M.D., Vice-Chair of Innovation and Medical Director, Electrophysiology Laboratory.
We have worked closely with leading patent experts to develop a robust IP strategy. Our IP strategic advisor – Sherpa Technology Group (STG) – is one of the best in the field, working at the intersection of business, technology, and intellectual property. Our patent attorneys, Sterne, Kessler, Goldstein & Fox, are equally distinguished and have been on the cutting edge of IP law for four decades.
We also have a technology development partnership with Plexus Corp. [Nasdaq: PLXS]. Their engineering and manufacturing capabilities and outstanding expertise in building complex medical devices make Plexus a foundational cornerstone to support BioSig’s growth objectives now and well into the future.
Key Growth Drivers
- 1) Advanced Technology — The non-invasive PURE EP System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring and calculating, displaying, recording and storing of electrocardiographic and intracardiac signals for patients undergoing electrophysiology EP procedures in an EP laboratory under the supervision of licensed healthcare practitioners who are responsible for interpreting the data. The PURE EP System aims to minimize noise and artifacts, and acquire high- fidelity cardiac signals. Improving cardiac signals may potentially increase the diagnostic value of these signals, thereby possibly improving accuracy and efficiency of the EP studies and ablation procedures.
- 2) Market Opportunity — 2019 DRG Medtech 360 Report states the global EP device market is expected to exceed more than US $10.1 billion by 2024 and is growing at a compound annual growth rate (CAGR) of 11.3%. The Company also operates within the rapidly emerging field of bioelectronic medicine, estimated at $25.11 billion in 2020 with projected annual growth of 10.27%.
- 3) KOL Support — PURE EP has been used and valued by many of the industry’s leading electrophysiology physicians, including Dr. Andrea Natale of Texas Cardiac Arrhythmia Institute, and Dr. G. Joseph Gallinghouse. The Company achieved proof of concept validation through UCLA, and has performed numerous pre-clinical studies at Mayo Clinic, MN under the leadership of Samuel J. Asirvatham, M.D., Mayo Clinic’s Vice-Chair of Innovation and Medical Director, Electrophysiology Laboratory.
Bioelectronic Medicine
Bioelectronic medicine is a rapidly growing field of healthcare that explores how targeted electrical signals can harness the body’s natural mechanisms to diagnose and treat a range of diseases. The field represents not just a narrow category of medical devices, but an entire approach to detecting and treating disease – using electrical pulses and the body’s own mechanisms as an adjunct or alternative to drugs and medical procedures.
Bioelectronic medicine applications aim to deliver treatment breakthroughs for many diseases that currently have a high level of unmet need. Researchers and innovators are exploring the field’s applications across various disease areas and disciplines, including neurology, auto-immune diseases, diabetes, arthritis, hypertension, pain management, cancer, and others. This wide range of applications sets bioelectronic medicine apart and indicates its immense potential.
We know we’re not alone in embracing a future with bioelectronic medicine. The field is making rapid strides, but this is just the beginning of what’s possible. That’s why we helped create the Alliance for Advancing Bioelectronic Medicine, an independent network of professionals dedicated to innovation at the intersection of healthcare and technology. We strive to develop this community with a common goal of realizing the field’s full potential.
Bioelectronic medicine is already a diverse, $20 billion market. It includes both familiar devices, such as pacemakers, as well as emerging technologies, such as vagus nerve stimulators and implantable neurostimulators. These exciting new segments are proliferating and attracting interest and investment from major players in technology and healthcare, such as Verily Life Sciences, Medtronic, and Johnson & Johnson.
As the field continues to develop, we believe our unique technology can play a critical enabling role. By providing more precise biomedical signals, our advanced signal processing capabilities can help clinicians better understand and change patterns to treat, or even prevent diseases.

NEWS
MANAGEMENT

Kenneth L. Londoner, MBA
Founder, Chief Executive Officer, Chairman, and Director
Mr. Londoner is a capital markets and capital architecture expert and a senior life science executive. Having started his career as a research analyst for J. & W. Seligman & Co., Inc., a leading institutional money management firm in New York City, NY, he quickly found himself at the forefront of the biotech industry in the early 1990s, leading him to manage $3.5 billion in mutual and pension funds and international assets.
His passion for medical innovation led him to co-found, govern and bring to the public market a number of life science companies, including BioSig Technologies, Inc. [Nasdaq:BSGM]. Working in close collaboration with key opinion leaders in electrophysiology, BioSig aims to improve the outcomes of cardiac ablations for the treatment of arrhythmias through novel technological solutions developed by the company, and further apply its core competency in advanced biomedical signal processing and analysis to other areas of medicine.
His prior experience in recognizing the early potential of biotech led Londoner to form the Alliance for Advancing Bioelectronic Medicine, an independent non-profit network of professionals dedicated to innovation at the intersection of healthcare and technology. The Alliance aims to increase awareness of bioelectronic medicine and build a platform for collaboration among stakeholders. Over the last decade, Londoner formed top level relationships with a number of medical centers of excellence such as Mayo Clinic, NYU Langone Hospital and UCLA, as well as other stakeholders, including investment communities, intellectual property experts and supply chain partners.

Steve Chaussy
Chief Financial Officer
Mr. Chaussy has served as Chief Financial Officer of BioSig Technologies, Inc., since May of 2011, making him one of the Company’s most veteran executives. He brings an impressive array of professional experiences, detailed protocols, and an adept understanding of the financial operations of public companies.
Mr. Chaussy has an extensive entrepreneurial growth and development background from working for Penske Truck Leasing Co., L.P., a Penske Corporation subsidiary, where his improvements of management and reporting tools allowed the Company to double in size. Since 2005, Mr. Chaussy has been the sole proprietor of Anna & Co., Inc., a consulting company that offers services to small publicly traded companies. Anna & Co., Inc. provides general financial and accounting services, emphasizing SEC reporting and compliance, to companies that lack sufficient resources to hire full-time employees to offer such services. From 2001 to 2005, Mr. Chaussy provided services as both a Chief Financial Officer and a consultant to small publicly traded companies. Before 2001, Mr. Chaussy served as the chief financial officer for a large private distribution and wholesaling Company, where he gained international experience.
Mr. Chaussy is a graduate of Virginia Polytechnic Institute and State University and is a licensed, certified public accountant in Virginia, California, and Florida.

Natasha Drapeau
Executive Vice President
Natasha Drapeau has been with BioSig Technologies, Inc. since May of 2017 and is primarily responsible for leading Company operations. Based in Geneva, Switzerland, Ms. Drapeau is BioSig’s primary executive for future European operations. She works closely with the Board of Directors and strategic partners, including medical centers of excellence and IP advisors. Ms. Drapeau serves as President at NeuroClear, Inc., Executive Vice President at BioSig Technologies, Inc., and is a founding member of the Alliance for Advancing Bioelectronic Medicine.
Ms. Drapeau brings over 18 years of international business development experience, including more than nine years at IG Group Plc, a leading British FTSE 250 financial services company. While working for IG, she was responsible for the Group’s global business development, including a worldwide rebranding and business support to 16 international offices in Europe, South Africa, Singapore, and Australia.
Mrs. Drapeau holds two MSc degrees with honors in Management and German from Samara Academy of Economics, as well as a Business, International Relations, and Political Economy degree from The London School of Economics. Mrs. Drapeau is a member of the Institute of Directors in the United Kingdom.

Brenda Castrodad
Director of Human Resources
A seasoned executive, Ms. Castrodad brings a wealth of experience in leading organizational development in start-ups and Fortune 500 companies within the life sciences sector. Most recently, Ms. Castrodad led the HR department at TissueTech, Inc., a Miami, FL-based biotech leader in regenerative amniotic tissue-based products, where she was responsible for transformation and automation of the company’s HR practices, talent planning, and team building. Prior to TissueTech, Inc., Ms. Castrodad spent six years at HeartWare, Inc., a heart failure medtech company acquired by Medtronic [NYSE:MDT] in 2016 for $1.1 billion. By optimizing the internal talent acquisition function and aligning business practices, Ms. Castrodad helped grow the organization from approx. 80 to 500+ staff which achieved approx. $250 million in revenues before the acquisition. Earlier in her career, Ms. Castrodad spent 16 years at Schering-Plough Corp, a pharmaceutical company acquired in 2009 for $41.1 billion by Merck & Co. [NYSE:MRK]. Ms. Castrodad holds a Master’s Degree in Public Administration and a Bachelor’s Degree in Social Sciences – Human Welfare from the University of Puerto Rico and a Labor Relations Certificate from the University of Michigan.

Andrew Ballou
Vice President, Investor Relations
Mr. Ballou brings to BioSig over 25 years of experience in capital markets, including institutional equity sales and research analysis. Most recently, Mr. Ballou served as Managing Director, Head of Institutional Equity Sales at Janney Montgomery Scott LLC., a role in which he oversaw key accounts, including large multi-manager hedge funds, mutual funds and dedicated sector funds. Prior to that role Mr. Ballou managed selected key account coverage at RBC Capital Markets, including Millennium Partners, Soros Fund Management, SIR Capital, Columbia Threadneedle, Two Sigma Investments and Times Square Capital Management. During the course of his career Mr. Ballou analyzed various private and public companies in healthcare, media and retail sectors. Mr. Ballou graduated from Hampden-Sydney College, Virginia, with a Bachelor of Arts in English.
Sincerely,
The Viral Stocks Team











